
Dive into the heart of lithium-ion batteries. In this blog, you’ll learn about the tiny parts inside. From anodes to cathodes, all play a big role. All these small pieces make up a battery. They allow it to store and give out power. Through this, you’ll gain knowledge about the very essence of lithium-ion batteries.
Components of a Lithium-Ion Battery!

Image Source: www.volts.wtf
§ Cathode
The cathode, a major player in lithium ion battery components, works hard. Made mostly of lithium cobalt oxide, or LiCoO2, the cathode holds lithium ions. While the battery charges, ions move from the anode to the cathode. In contrast, during use, ions head back to the anode. Materials such as LiNiO2 or LiMn2O4 are also common. The variety in material affects battery performance.
§ Anode
The anode of a lithium-ion battery is typically graphite. Here, lithium ions nestle between layers during charging. Carbon, the main element in graphite, excels in storing lithium ions. When the battery is in use, ions move to the cathode. Silicon-based anodes have higher capacity, but wear out quickly.
§ Electrolyte
In the middle of a battery, there’s the electrolyte. This is a liquid filled with lithium ions. With high conductivity, it permits ions to travel between the cathode and anode. The electrolyte must be stable and not react with the battery’s other parts. Hence, complex lithium salts in organic solvents are often used.
§ Separator
A thin piece, the separator, keeps anode and cathode apart. However, it lets lithium ions pass freely. Polypropylene or polyethylene is common materials for separators. The separator prevents short circuits, avoiding battery fires. The integrity and safety of a battery highly depend on the separator.
§ Current Collectors
At the cathode, an aluminum foil collects the current. On the other hand, copper foil serves the anode. These foils conduct electricity from the electrodes out of the battery. Foil thickness, surface treatment and cleanliness all affect battery performance.
§ BMS
BMS checks the battery health and safety. It manages the charging process. The goal is to extend battery life. The BMS will shut down the battery if it gets too hot or cold. It also avoids overcharging and deep discharging.
§ Cathode
The cathode is usually made of lithium metal oxide. During the battery charging, lithium ions move from the cathode to the anode. The cathode materials affect the battery’s voltage and energy capacity. Cobalt oxide is a common choice for cathodes. It gives the battery high energy density.
§ Anode
The anode is usually made of carbon, like graphite. When the battery discharges, lithium ions move back to the cathode. The process helps release energy. The size and structure of the anode impact battery performance.
§ Lithium Salts
These salts form the electrolyte. The electrolyte is a bridge for lithium ions. They move between the cathode and anode through this bridge. A common lithium salt is lithium hexafluorophosphate (LiPF6). It works well in organic solvents.
§ Organic Solvents
These solvents make the liquid electrolyte. They carry the lithium ions between the anode and cathode. The solvent choices impact the safety and performance of the battery. A mix of ethylene carbonate and diethyl carbonate is often used. The mixture helps the lithium ions move smoothly.
§ Protection Circuit
Protection Circuit’s role is to keep batteries safe. Should voltage reach beyond 4.2 volts, or below 2.5 volts, it intervenes. Acting like a guard, it halts the charging or discharging process. This ensures safe and reliable battery operation.
§ Electrode Particle Size
Moving on, consider the Electrode Particle Size. It’s significant in lithium ion batteries. Big particles slow down ion movement. This, in turn, slows charging and discharging. On the contrary, small particles lead to better performance. However, making particles too small could risk battery stability.
§ Shell or Casing
The casing encloses the battery’s insides, safeguarding them from harm. In addition, it provides structural stability to the battery. Made from metal, the casing withstands pressure changes inside the battery. Plus, it helps dissipate heat, aiding in maintaining the battery’s optimal temperature.
§ Cell Arrangement
Cell Arrangement refers to how the cells within the battery are organized. Common arrangements include prismatic, cylindrical, and pouch cells. Each type has specific pros and cons, impacting energy density, cost, and thermal management.
§ Electrode Formulation
Electrode Formulation is a mix of active material, conductive additive, and binder. For anodes, graphite is a common active material. For cathodes, a mix of lithium and other metals is common. The formulation influences the lithium ion battery working, impacting energy density, power density, safety, and life span.
| Component | Function | Material Used | Importance (1-10) | Vulnerability to Damage (1-10) | Role in Energy Transfer | Related Terms |
| Anode | Stores lithium ions | Graphite | 8 | 7 | Releases ions during discharge | Lithiation, Delithiation |
| Cathode | Accepts lithium ions | Lithium Cobalt Oxide | 8 | 6 | Accepts ions during discharge | Intercalation, Deintercalation |
| Electrolyte | Conducts ions | Lithium Salt in Organic Solvent | 9 | 8 | Facilitates ion movement | Ion Conductivity |
| Separator | Prevents short circuit | Polyethylene, Polypropylene | 10 | 7 | Prevents direct contact of anode and cathode | Dielectric |
| Current Collectors | Conducts electrons | Copper (anode), Aluminium (cathode) | 8 | 5 | Facilitates electron flow | Electron Conduction |
| Safety Vent | Pressure release | Various metals and plastics | 6 | 4 | Ensures safe operation | Venting, Rupture Disk |
| Positive Terminal | Current outflow | Metal (usually steel) | 7 | 4 | Outflow of current during discharge | Cathode Terminal |
| Negative Terminal | Current inflow | Metal (usually copper) | 7 | 4 | Inflow of current during charge | Anode Terminal |
| Battery Management System | Protects and manages battery | Electronic Circuits | 10 | 8 | Controls charge, discharge, and temperature | BMS, SOC, SOH |
Table on Components of a Lithium-Ion Battery!
Battery Construction and Assembly!
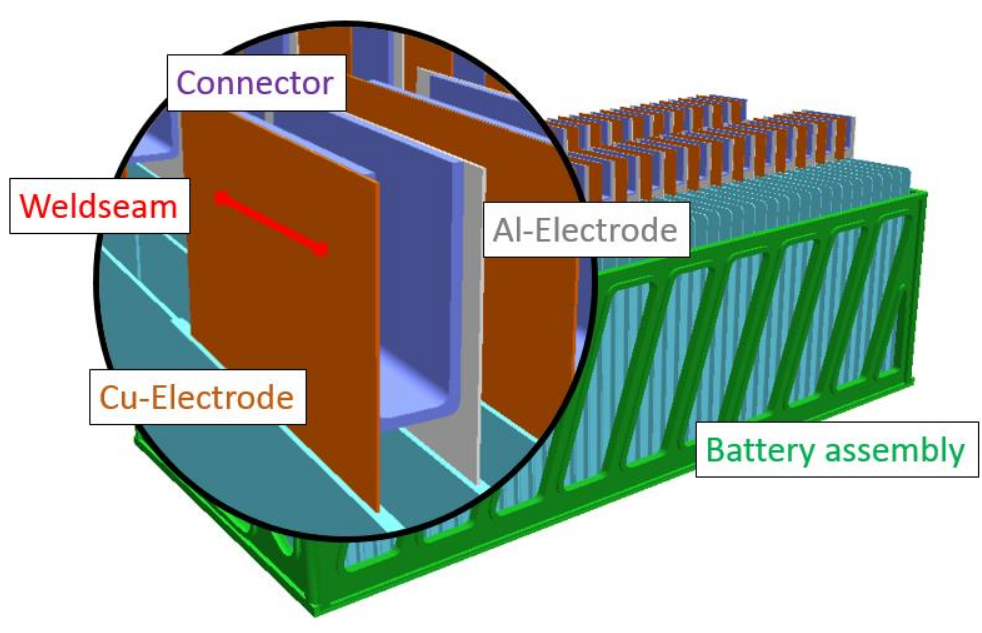
Image Source: www.researchgate.net
§ Electrode Preparation
In Lithium Ion Battery Components, electrode preparation stands vital. Initially, paste is made with active material, binder, and conductive agent. A machine then spreads this paste evenly on a metal foil, forming the electrode. This coated foil undergoes compressing and drying, leaving a thin, uniform layer. Typically, one electrode has lithium; the other is carbon or graphite.
§ Separator Insertion
Once electrodes are ready, a separator is inserted. The separator is a thin sheet, typically made from polyethylene or polypropylene. Its purpose is to keep the electrodes apart, preventing a short circuit. Precision is key in this step. The separator must cover the entire area, but cannot touch the edges, to avoid internal short circuits.
§ Electrolyte Injection
Now comes the part of the lithium ion battery electrolyte. This liquid serves as the medium for lithium ions to move between electrodes. Proper filling is critical. Too little, the battery performance drops. Too much, it may lead to leaks.
§ Cell Sealing
After electrolyte injection, the cell undergoes sealing. The battery cell housing, usually aluminum or steel, is hermetically sealed. Next, a safety valve is installed, allowing for controlled venting if the internal pressure rises too high. Proper sealing ensures the safety and longevity of the battery.
§ Formation Process
Here, the battery gets its first charge and discharge cycles at a controlled rate. These initial cycles form a protective layer on the electrodes, critical for battery performance. Detailed monitoring takes place, checking voltage and temperature, guaranteeing each battery leaves the factory ready for safe and efficient use.
§ Safety Vent Installation
A safety vent forms a critical part of the Lithium Ion Battery Components. It is put in place for preventing battery damage. While pressure builds up inside the vent opens. Excess pressure leaves through the vent. The pressure usually comes from overheating. Battery temperatures over 100 degrees Celsius trigger the vent. Keeping the battery safe is the main goal.
§ Cathode Layering
This is where the positive charge lives. Lithium ions move from here during discharge. The layering process is quite precise. It involves coating an aluminum foil with cathode material. Common materials are Lithium Cobalt Oxide (LCO) or Lithium Iron Phosphate (LFP). Then, it goes through a drying phase.
§ Anode Layering
Similar to the cathode, layering is done here. The process involves coating a copper foil. The coating material is usually graphite. Sometimes, silicon is also used. Once coated, it goes through drying and compression. The anode is now ready. It stores lithium ions during charging.
§ Stacking
Both anode and cathode are brought together. A separator is placed in between. This prevents any direct contact. Contact could cause a short circuit. Therefore, the separator is vital. Then, layers are stacked on top of each other. This forms the battery cell.
§ Encapsulation
The stacked cells are enclosed. A strong, durable casing is used. It protects the delicate insides from harm. This casing could be made from metal or plastic. The casing provides an essential seal. It keeps out dust and moisture. This makes the battery safe for everyday use.
§ Cell Formation
Cells come from layers: anode, cathode, and a separator. Wrapped in rolls, these layers form a jelly roll design. Anode uses graphite; cathode uses lithium compounds, and separator uses thin plastic. These bits make sure the power flows safely.
§ Battery Casing
Once the cells are ready, they need a home. This is the battery casing. Usually made of light durable aluminum. Its job is to guard the cells, keeping them safe and secure. Imagine a protective shell for your precious cells. That’s what a casing does.
§ Cell Connection
Now, cells must connect. Connections made by thin, metal tabs. One end attaches to the cell, the other to the battery terminal. This creates a bridge for power. It lets energy flow from cell to device.
§ Quality Inspection
Engineers use advanced equipment, checking every bit. They look at voltage, capacity, and internal resistance. If a problem shows, they find and fix it.
§ Packaging
Safe, sturdy boxes are a must. Protection against damage during shipping is important. Each box can hold multiple units. All set to reach the hands of users.
Lithium-Ion Battery Performance Parameters!
§ Energy Density
High energy density means more stored energy. Imagine a small box. If full of energy, it powers long drives in electric cars. Thus, energy density is a vital parameter. For example, one common lithium-ion battery might have 150 Wh/kg (watt-hours per kilogram). That’s a lot of energy! So, energy density is a big deal in battery design.
§ Power Density
More power density equals faster energy release. Think of a fast electric car. Speed needs quick energy. Hence, high power density is a must. A battery, like the popular LiCoO2, can have a power density of around 1500 W/L (watts per liter). More power density leads to quicker charging and more powerful machines.
§ Discharge Rate
The discharge rate tells how fast a battery drains. A higher rate means quicker energy use. For instance, a 1C discharge rate uses all energy in one hour. Lower rates like 0.5C extend battery life to two hours. Thus, the discharge rate affects battery life. Engineers must consider this rate when creating effective batteries.
§ Cycle Life
A cycle is one full battery charge and discharge. After many cycles, batteries lose power. The more cycles a battery can withstand before power loss, the longer its life. For instance, some lithium-ion batteries can handle 500 to 1000 cycles. Greater cycle life equals longer battery life.
§ Charge Rate
Speed in filling a battery with power is crucial. Often measured in ‘C’, the charge rate denotes how quickly a Lithium-Ion battery gets full. For instance, a 1C rate means the battery charges fully in an hour. High charge rates may shorten battery life. Conversely, lower rates enhance longevity.
§ Open-Circuit Voltage
Imagine a battery as an untapped energy reservoir. Open-Circuit Voltage (OCV) is the battery’s potential energy, ready to be used. Without any load connected, OCV equals the full voltage. In lithium-ion batteries, OCV usually ranges between 3.6 to 3.7 volts. OCV determination aids in evaluating a battery’s state of charge (SoC).
§ Load Resistance
In simple terms, load resistance refers to the opposition faced by current in a circuit. The value impacts battery output, measured in ohms (Ω). Lower resistance means higher current, leading to faster energy discharge. Conversely, higher resistance slows down the discharge.
§ Self-Discharge
Every battery loses energy over time, even when not in use. Self-discharge denotes this energy loss rate. For lithium-ion batteries, the monthly self-discharge sits around 1.5-2%. Too high self-discharge renders a battery unfit for long-term storage, impacting the reliability of the components.
§ Battery Efficiency
Efficiency reveals how well a battery converts input power to useful output. Measured as a ratio, it often reaches 80-90% in lithium-ion batteries. Greater efficiency translates to less waste, making the battery a reliable energy storage device. Battery efficiency, hence, serves as a vital parameter in assessing the effectiveness of the components.
§ Operating Temperature
Too high or too low can harm the battery. For example, less than 20 degrees Celsius or over 60 degrees Celsius can cause damage. So, keeping the temperature in a safe range is essential. Experts often suggest a safe zone between 20 and 25 degrees Celsius for optimum battery performance.
§ Voltage Hysteresis
In simple terms, it is the difference in voltage when a battery charges and discharges. A smaller difference means better efficiency. An ideal value for voltage hysteresis is about 0.1 volts. Remember, less is more when it comes to voltage hysteresis.
§ Safety Threshold
The safety threshold of a battery defines its resilience. It is the upper limit a battery can withstand before its structure or performance deteriorates. For Lithium-Ion batteries, the safety threshold is about 4.2 volts per cell. Going above this value can lead to significant issues, such as overheating or even explosion.
§ Peak Power
Peak power determines the maximum energy a battery can deliver at once. For Lithium-Ion batteries, this value ranges from 1500 to 3000 watts per kilogram. This amount of energy can power an electric vehicle or a laptop easily. Keeping the peak power in check ensures your device gets the right amount of energy when needed.
§ Battery Capacity
Battery capacity shows how much energy a battery can store. For a typical Lithium-Ion battery, capacity ranges from 1000 to 3000 milliamp-hours (mAh). The higher the capacity, the longer the battery can power a device.
§ Storage Temperature
Ideal storage temperatures for Lithium-Ion batteries range from 10 to 25 degrees Celsius. Storing batteries at such temperatures helps maintain their capacity and prolongs their life. Being aware of the right storage temperature can help optimize battery usage over time.
The Role of Lithium in Lithium-Ion Batteries!
§ Ion Movement
In the realm of lithium ion battery chemistry, lithium ions play a leading role. Ions hop from the anode to the cathode during discharge. On charging, ions bounce back to the anode. This back and forth marks a key feature of Lithium Ion Battery Components, creating a smooth energy flow.
§ Voltage Production
The difference in potential between anode and cathode produces voltage. The materials used for these parts decide the voltage value. Typical voltage for a lithium-ion cell measures around 3.7 volts.
§ Energy Storage
The design of lithium-ion batteries ensures high capacity. The amount of lithium ions the cathode can absorb dictates capacity. More absorption equals greater storage. So, by adjusting the cathode material, one can change a battery’s capacity.
§ Discharge Mechanism
During discharge, lithium ions migrate from the anode to the cathode. They traverse the electrolyte, contributing to electric current. The speed of this migration affects the battery’s discharge rate.
§ Charge Mechanism
When charging, lithium ions move in reverse. They shift from the cathode back to the anode through the electrolyte. The charger provides the energy for this uphill move, refilling the battery for another cycle.
§ Interlayer Diffusion
Anodes and cathodes comprise layered structures. In these layers, lithium ions find their way. These ions maneuver around, showcasing their skill at interlayer diffusion. This characteristic impacts the charge and discharge rates.
§ High Energy Density
One of the triumphs of lithium-ion batteries is their high energy density. They store more energy in less space. In a standard cell, you’ll find an energy density above 150 Wh/kg. Such power in a small package makes lithium-ion batteries ideal for portable devices.
§ Electrochemical Reaction
In lithium-ion battery chemistry, lithium ions shift from the negative electrode to the positive during discharge. Then, reverse back during charging. Moving ions create electric current. This current powers devices. Power is based on ion movement speed.
§ Reversibility
Key to the success of lithium-ion batteries is reversibility. Lithium ions can move back and forth between the electrodes. That’s what happens during charging and discharging. The process is like a dance. The ions dance back and forth, creating power each time.
§ Stable Cycling
Each lithium-ion battery’s life relies on stable cycling. Imagine cycling as a marathon. In each cycle, lithium ions run from one electrode to the other. The more cycles the battery can handle, the longer the battery lasts.
§ Oxidation Process
When charging, lithium ions leave the positive electrode. The electrode loses electrons. This process is oxidation. It’s like a basketball game. The electrode passes electrons to the battery.
§ Reduction Process
On discharging, the positive electrode gains electrons. That’s called reduction. Electrons return like boomerangs. The more efficient this process, the more power the battery provides.
§ Lithium Plating
A downside in lithium-ion batteries is lithium plating. Here, lithium builds up on the negative electrode. Like a dam, it blocks ion movement. This reduces battery life and can pose safety risks.
§ Ion Conduction
Ion conduction is vital. It’s the heart of a lithium-ion battery. The electrolyte plays a major role. It guides the ions, like a coach guiding players in a game.
§ Electrolyte Interaction
Last, the electrolyte’s interaction with the electrode materials is crucial. It’s like two dance partners. If they work together well, the performance is impressive. If not, things can go wrong, causing shorter battery life or even safety issues.
Detailed Overview of Anode Materials!
§ Graphite
At the core of lithium ion battery construction and working lies Graphite, the main anode material. With a capacity of 372mAh/g, graphite holds lithium ions during charging. Carbon atoms, tightly arranged, form a layer-like structure. Here, lithium ions find a home. Despite the low voltage, graphite’s high energy density proves valuable.
§ Lithium Titanate
A shift from graphite brings us to Lithium Titanate. Featuring a 3D crystal structure, lithium titanate welcomes more lithium ions. Its fast charging property sets it apart. The LTO anode, with 175mAh/g capacity, resists damage during fast charging. High-temperature stability and longer cycle life highlight its advantages.
§ Silicon
In the search for high-capacity anodes, Silicon emerges. A silicon anode can house 4,200mAh/g capacity, impressive in comparison. But, there’s a catch! Silicon swells during charging, leading to cracks. Solving the silicon-swelling puzzle remains a challenge for scientists.
§ Hard Carbon
Next in line is Hard Carbon. More disorderly than graphite, its structure is a lithium-ion haven. With capacities reaching up to 1,000mAh/g, hard carbon promises higher energy density.
§ Lithium
Pure Lithium carries the potential for high capacity. With 3,860mAh/g, lithium surpasses other anode materials. Yet, safety risks linked with metallic lithium make its application difficult.
§ Alloy-based Materials
Alloy-based materials like silicon alloys are promising candidates. They tackle the silicon swelling issue and improve overall stability. Yet, the lower electrical conductivity of alloys needs addressing.
§ Particle Size
Anode materials’ particle size influences battery performance. Smaller particles offer more surface area for lithium-ion interaction. But, tiny particles may clump together, obstructing ion flow. A balance in size brings optimal battery function.
§ Material Purity
The purity of anode materials bears significance. Contaminants can alter the anode’s behavior. To achieve high performance, material purity should remain a priority. Thus, the battery industry maintains strict purity control.
§ Surface Coating
Coatings on anode materials play a vital part in Lithium Ion Battery Components. Tiny layers, usually 2-4 nm thick, protect the anode. Protective coatings include Al2O3 or ZrO2. With them, battery safety increases.
§ Interfacial Stability
Interfacial stability matters. Anode and electrolyte interaction should stay stable. Li-ion batteries have graphite anodes. These need electrolyte stability. Without it, SEI layers form. So, failure in performance can occur.
§ Volume Expansion
A key issue with anode materials is volume expansion. Silicon anodes expand and contract. Up to 300% changes occur during charging and discharging. Therefore, unwanted side reactions and cracking happen, lessening battery life.
§ Material Morphology
Another vital aspect is material morphology. Anode structure impacts Li-ion battery performance. For example, porous silicon provides more space. This space accommodates volume changes, enhancing stability.
§ Conductivity
Anode conductivity plays an essential role. Low conductivity means slow charging and discharging. Copper, due to high conductivity, gets used as the anode’s current collector.
§ Cycle Stability
Cycle stability ensures long-term battery use. A high number of cycles is a desired trait. After 500 cycles, capacity should retain 80%. Graphite anodes meet this need.
§ Layer Structure
The layer structure of graphite anodes allows lithium insertion. The layers accommodate lithium ions during the charging phase. Therefore, the layering in anodes greatly influences the battery’s efficiency.
Detailed Overview of Cathode Materials!
Image Source: www.nature.com
§ Lithium Cobalt Oxide (LiCoO2)
LiCoO2 is used in lithium-ion batteries (LIBs). It’s a vital part. As a cathode material, it has a high energy density of 150-200 Wh/kg. However, it’s expensive. LIBs with this component need careful handling. Safety issues arise due to the material’s instability.
§ Lithium Manganese Oxide (LiMn2O4)
LMO is another key cathode material. This material’s use in LIBs boosts power density. Its structure gives high thermal stability. It’s less toxic than LiCoO2, making it safer. Yet, the energy density (100-120 Wh/kg) falls short of LiCoO2.
§ Lithium Iron Phosphate (LiFePO4)
LFP is a popular cathode material. In LIBs, LFP gives a stable performance. It has high thermal stability. The energy density is about 90-120 Wh/kg. The low cost and safety factor are advantages. Yet, it has lower conductivity.
§ LiNiMnCoO2 or NMC
NMC is a mixed cathode material. It offers a balance of power and energy density. The energy density reaches 140-220 Wh/kg. It’s popular in electric vehicles (EVs).
§ Particle Morphology
Particle size and shape matter in cathode materials. Uniform particle size boosts performance. The aim is to achieve ideal particle morphology. This can reduce resistance. It can also enhance ion transfer.
§ Material Purity
Pure materials enhance battery performance. Any impurity can affect the capacity and life of LIBs. High purity of cathode materials is thus vital. Industries strive for maximum purity in the materials.
§ Surface Coating
Surface coating improves cathode material performance. It increases thermal stability. It also protects the cathode material. Common coatings include Al2O3 and ZrO2. The coating method impacts the final performance.
§ Thermal Stability
Cathode materials need thermal stability. It’s crucial for safety. High thermal stability reduces the risk of battery failure. The search is on for materials with higher thermal stability.
§ Crystal Structure
In the heart of lithium-ion batteries (LIBs) is the crystal structure. Here, the key is in the orderly arrangement of atoms. Materials like LiCoO2, LiMn2O4, and LiFePO4 are common. Each provides unique benefits. LIBs require high structural integrity. High-quality crystal structures ensure top performance.
§ Conductivity
Now, we’re on to conductivity. This measures how lithium ions move in the battery. Higher conductivity means better energy flow. LFP (LiFePO4) is a good example. It boasts high conductivity. This results in quick charging and longer life.
§ Voltage Plateau
The voltage plateau is essential. It is the stable voltage during discharge. LIBs with higher plateaus can store more energy. Like in NMC (LiNiMnCoO2) batteries. It has a plateau of 3.7 volts. This means more energy for your devices.
§ Energy Density
Energy density is next. It relates to how much energy a battery can hold. LIBs like NCA (LiNiCoAlO2) have high energy densities. With an energy density of 200 Wh/kg, NCA batteries can power your device for hours.
§ Cycle Stability
Cycle stability is the battery’s lifespan. It measures the battery’s charge and discharge cycles. LCO (LiCoO2) batteries can go up to 1000 cycles.
§ Rate Capability
Rate capability tells us about the battery’s power output. High rate means quick discharge. LMO (LiMn2O4) excels here. It can deliver high power fast. This is crucial for devices that need quick energy, like electric cars.
§ Layer Structure
It is the way atoms are stacked in the battery. Layered structures like in NCA batteries allow for easy lithium movement. This helps with conductivity and power output.
Detailed Overview of Electrolyte Materials!
§ Lithium Hexafluorophosphate (LiPF6)
LiPF6 is key in Li-ion batteries (LIBs). This material, due to its ionic conductivity, aids energy transfer. It’s stable up to 70°C. However, above this temp, it breaks down.
§ Lithium Tetrafluoroborate (LiBF4)
Similar to LiPF6, it enables energy flow. However, it boasts superior thermal stability. It can endure up to 150°C. Thus, it may provide a more durable and safer battery.
§ Lithium Perchlorate (LiClO4)
LiClO4, used in early LIBs, offers high conductivity. Yet, it’s thermally unstable, degrading at 130°C. This property limits its use due to safety concerns.
§ Organic Solvents
Organic solvents in LIBs carry lithium ions. Common ones include EC, DMC, and DEC. They enable efficient energy transfer. However, they have flammability issues.
§ Ionic Conductivity
It refers to the ability of ions to move through a material. In LIBs, high ionic conductivity ensures efficient energy flow. Materials like LiPF6 and LiBF4 are used to enhance this property.
§ Electrochemical Stability
This feature impacts the safety and lifespan of LIBs. Materials with high electrochemical stability, like LiBF4, resist breakdown.
§ Viscosity
This factor affects the ionic conductivity of the electrolyte. Lower viscosity enhances ion movement, leading to improved battery performance.
§ Dielectric Constant
This metric measures a material’s ability to store electrical energy. High dielectric constants in electrolytes can improve energy storage and transfer in LIBs.
§ Lithium Cobalt Oxide (LCO)
LCO is a vital part of lithium-ion batteries (LIB). It’s found in the cathode. LCO has high energy density. Its structure is layered. However, it poses safety risks under certain conditions. To use it effectively, safety measures are crucial.
§ Lithium Manganese Oxide (LMO)
In the cathode, LMO plays a significant role. LMO offers good thermal stability. It’s ideal for power tools and electric vehicles (EV). However, it has lower energy density.
§ Lithium Iron Phosphate (LFP)
LFP offers thermal and chemical stability. It makes batteries safer. Plus, it extends battery life. Its energy density is less than LCO. But it’s the top choice for large-scale systems.
§ Lithium Nickel Manganese Cobalt Oxide (NMC)
NMC is a mix of nickel, manganese, and cobalt. You’ll find it in the cathode. NMC offers a balance. It provides high energy density and stable chemistry. Thus, it’s used in EVs and grid storage.
§ Particle Morphology
This is about the shape and size of cathode materials. It affects the performance of LIBs. Smaller particles can deliver energy faster. Yet, they reduce the battery’s lifespan. So, it’s all about balance. Proper particle morphology ensures optimal battery performance.
§ Material Purity
Purity of the cathode material is key. Even tiny impurities can harm battery performance. Higher purity leads to longer lifespan and better safety. So, manufacturers work hard to ensure material purity.
§ Surface Coating
Surface coating can enhance cathode materials. It prevents side reactions. That way, it extends battery life. It also improves safety and efficiency. In short, surface coating plays a vital role in LIB manufacturing.
§ Thermal Stability
Thermal stability ensures the battery can withstand high temperatures. This prevents overheating and potential failures. Hence, choosing materials with high thermal stability is crucial for safe and reliable LIBs.
Detailed Overview of Separator Materials!
§ Polyethylene
In lithium-ion batteries (LIBs), polyethylene (PE) reigns supreme. PE, a separator material, is often 20 micrometers thin. Its role, paramount. It averts direct contact between positive and negative electrodes. LIB failure could ensue without it. PE, with its sturdy structure, is highly resistant to shrinkage at temperatures up to 130°C. High melting point is another boon.
§ Polypropylene
In LIBs, it’s another prevalent separator material. Slightly thicker than PE, about 25 micrometers, it’s sturdier. It resists heat well. It can withstand temperatures up to 150°C. So, LIBs gain an extra layer of protection.
§ Ceramic Coating
Al2O3 or SiO2 are typical ceramic materials used. It enhances wettability, reduces shrinkage, and improves thermal stability.
§ Pore Size
Pore size typically varies between 0.01 to 1 micrometer. The size affects ion flow. So, right size means smooth flow, resulting in optimal battery function.
§ Thickness
The separator’s thickness is between 20-30 micrometers. The aim is to maintain battery safety while allowing free ion movement.
§ Wettability
Separator wettability ensures smooth electrolyte diffusion. Poor wettability leads to uneven ion flow, disrupting LIB performance.
§ Thermal Stability
LIB separators need to be thermally stable. It enables them to resist high operating temperatures. Materials like PE, PP, and ceramic coating provide this much-needed stability.
§ Mechanical Strength
A strong separator withstands physical stress during LIB operation. So, it’s a vital factor in ensuring LIB’s long-term performance.
§ Insulation Properties
The separator’s insulation properties prevent a direct electrical connection between the anode and cathode. This deters any unwanted current flow. In LIBs, a separator made of polyethylene (PE) or polypropylene (PP) often fits the bill. The separator should have high chemical stability.
§ Ionic Resistance
Ionic resistance influences energy density and charge-discharge rates. A lower ionic resistance results in a higher conductivity for lithium ions. This boosts the battery’s power output. The goal is to achieve as low an ionic resistance as possible.
§ Electrolyte Uptake
A separator must absorb the electrolyte well. This aspect is known as electrolyte uptake. It directly affects ionic conductivity. The separator absorbs the liquid electrolyte in a LIB. Polyolefin-based separators do this job well. Their high electrolyte uptake enhances the battery’s performance.
§ Shutdown Feature
The separator also has a shutdown feature. In overheating scenarios, the separator melts and closes its pores. This stops the ion flow, thus preventing a thermal runaway. It is a built-in safety mechanism in LIBs.
§ Pore Distribution
The separator’s pore distribution affects ionic conductivity and mechanical strength. An even pore distribution in the separator ensures efficient ion flow. Most separators have pore sizes ranging from 50 to 200 nanometers.
Battery Capacity and Energy Storage!

Image Source: www.mercomindia.com
How capacity is measured?
Capacity in a battery is measured in milliampere-hours (mAh). It shows how much energy a battery can hold. Higher mAh means more energy.
It’s like a big water tank that can hold lots of water. In a Lithium Ion (Li-Ion) battery, key parts are the anode, cathode, separator, and electrolyte.
The anode and cathode hold the lithium ions. The separator keeps them apart, but let’s ions move. The electrolyte carries ions from the anode to the cathode, and back.
Factors that boost battery capacity
o Cathode Material
Li-Ion batteries often use Lithium Cobalt Oxide (LCO) for the cathode. It’s because LCO can store lots of lithium ions. More ions equal more energy. But, new materials like Lithium Nickel Manganese Cobalt Oxide (NMC) are also used now. NMC can hold even more ions.
o Anode Material
Graphite is a common anode material in Li-Ion batteries. It’s good at holding lithium ions. But, Silicon (Si) is gaining interest. Si can hold more ions than graphite. Yet, Si expands a lot during charging. This can damage the battery.
o Electrolyte Composition
The electrolyte in a Li-Ion battery needs to be stable and safe. It often includes Lithium Hexafluorophosphate (LiPF6). The electrolyte carries the lithium ions. A good electrolyte can make a battery safer and last longer.
o Temperature
Li-Ion batteries work best at around 20 to 25 degrees Celsius. Too hot or too cold can harm the battery. Keeping a battery at the right temperature can boost its life.
o Charging Method
How a Li-Ion battery is charged matters a lot. Charging too fast can harm the battery. A slow, steady charge is best. This way, the battery can last longer.
o Discharge Rate
This is how fast energy leaves a battery. A high discharge rate can drain a battery fast. It can also harm the battery. A slow, steady discharge is best for a Li-Ion battery.
o Material Purity
In lithium-ion batteries, pure lithium (Li) plays a crucial role. This element holds three electrons, making it ideal for high energy storage. However, pure Li is reactive. Hence, Li-ion batteries use lithium compounds, with a typical Li-ion cell storing 150 Wh/kg.
o Electrode Design
The cathode in Li-ion batteries, often made from LiCoO2, is pivotal. It manages lithium ions during the charge and discharge process. Efficient designs can enhance battery life by 10-15%.
o Cell Construction
Li-ion batteries consist of an anode, cathode, and electrolyte. The anode, typically graphite, hosts Li ions during charging. The cathode, usually LiCoO2, releases Li ions during use. Separators prevent short circuits.
o Cell Size
The dimension of a Li-ion cell impacts its performance. A common 18650 cell, 18mm in diameter and 65mm long, can store up to 3500 mAh. Larger cells may offer more capacity, but with a risk of faster heat buildup.
o Particle Size
Particle size in electrodes affects Li-ion battery performance. Small particles, typically below 20 micrometers, can increase energy density. However, they can also intensify heat generation and lead to a shorter lifespan.
o Electrode Thickness
The thickness of the electrode in a Li-ion battery matters. Typically, anode thickness falls in the 50-60 µm range, while cathodes are around 70-80 µm. Thicker electrodes can store more energy but may limit ion movement.
o Current Collector
Current collectors, usually made from copper (anode) and aluminium (cathode), conduct electricity in the cell. Their design affects battery efficiency. Thickness and material selection are crucial factors here.
o Aging Effects
Over time, Li-ion batteries experience capacity loss due to aging. After 500 cycles, a battery may retain only 70% of its original capacity. High temperatures and overcharging accelerate aging effects.
Battery Efficiency and Performance Ideas!
§ Fast Charging
In lithium-ion battery components, fast charging is key. A 60-minute charge delivers 70% capacity. Consider a component like anode, made from graphite. Its role in fast charging is significant.
§ High Discharge
High discharge rates rely on the cathode, typically built with lithium cobalt oxide. High discharge allows energy release in short bursts, crucial in some applications.
§ Cycle Life
A high-quality Li-ion battery endures over 1,000 charge-discharge cycles. Performance hinges on a balanced electrolyte, ensuring ion transfer.
§ Wide Temperature Range
A wide temperature range enhances usability. Lithium-ion batteries can operate from -20°C to 60°C. Thermal management systems help maintain safe operating temperatures.
§ High Energy Density
Energy density indicates energy stored per unit volume. With 150-200Wh/kg, lithium-ion batteries stand out. Both anode and cathode materials significantly contribute to high energy density.
§ High Power Density
Power density, or energy delivered per unit time, is strength of lithium-ion batteries. With 300-500W/kg, they’re well-suited for high-drain devices. Again, anode and cathode matter.
§ Material Optimization
Material optimization ensures components work efficiently. For instance, reducing anode thickness increases energy density. So, refining each component is crucial.
§ Safety Measures
With mechanisms like pressure vents and thermal interrupt devices, lithium-ion batteries are protected from overheating and short circuits. Quality separators contribute to safety.
§ Improved Anode
An improved anode increases battery efficiency. Silicon-based anodes, for example, boost energy storage. Anode improvements add to battery performance and lifespan.
§ Improved Cathode
A high-quality cathode boosts battery performance too. For instance, lithium iron phosphate cathodes improve safety. Cathode enhancements drive efficiency, power, and safety.
§ Advanced Electrolyte
An advanced electrolyte can boost efficiency. Solid electrolytes, for example, enhance safety and longevity. They also maintain high ion conductivity, essential for battery operation.
§ Better Separator
A good separator aids battery safety and efficiency. Ceramic-coated separators resist high temperatures. They keep the anode and cathode separate, preventing short circuits.
Tips on Battery Life and Cycle Life!

Image Source: spectrum.ieee.org
§ Optimal Charging
Charging Lithium Ion Battery Components between 20-80% capacity often leads to improved battery health. Full 0-100% charging can cause stress on the anode and cathode.
§ Temperature Management
Too high or low temperatures affect battery performance. Keep batteries between 20-25°C (68-77°F) for top-notch efficiency. Control systems aid in maintaining the right temperature.
§ Overcharge Prevention
High voltages cause lithium ions to react excessively, affecting the electrode. Advanced Battery Management Systems (BMS) prevent overcharging.
§ Undercharge Prevention
Undercharging also harms the battery. BMS tech plays a role in averting this, ensuring appropriate charge levels.
§ Avoiding High Current
High current accelerates wear and tear. As a rule, less current equals longer battery life. Respect device specifications on current limits.
§ Minimizing Vibration
Excessive shaking or vibration could damage battery structure. Secure installations reduce the risk.
§ Regular Inspection
Regular checks catch faults early. Look for bulges, leaks, and signs of overheating.
§ Battery Resting
Rest periods after charging extend battery life. It allows redistribution of ions in the electrolyte.
§ Low Discharge Rate
A lower discharge rate contributes to longer lifespan. High discharge rates may lead to shorter battery life.
§ Safe Storage
Store the batteries in a cool, dry place. Avoid exposure to heat and moisture to prevent damage.
§ Capacity Maintenance
Keep track of battery capacity. Reduced capacity indicates wear, calling for a replacement.
§ Depth of Discharge (DoD)
Lower DoD increases battery cycle life. Aim to use less than 50% capacity before charging.
§ Charge Cutoff
Stop charging once the battery reaches its capacity. Overcharging is detrimental to battery health.
§ Use within Specification
Every battery has its specified use. Stick to the manufacturer’s guidance to ensure a longer life.
Battery Safety and Stability!
Common safety issues
o Thermal Runaway
Energy stored in a lithium-ion battery can overheat. When heat goes past 150°C, the battery’s cells may explode. This dangerous event is called thermal runaway.
o Overcharging
With overcharging, the battery’s voltage is too high. High voltage can cause the battery to catch fire. A well-designed battery management system helps to avoid such situations.
o Short Circuit
A short circuit happens when electricity bypasses the normal path. This can make the battery overheat or even explode. Fuses and circuit breakers offer protection against short circuits.
o Mechanical Damage
Physical harm can lead to battery leaks or fires. Hence, batteries require sturdy, damage-resistant casings.
o Swelling
Gas buildup causes battery swelling. It’s often due to overcharging or exposure to high temperatures. Regular checks help to detect swelling early.
o High Temperature
Overheating harms lithium-ion batteries. The upper limit is 60°C for most models. Always keep batteries in a cool place.
o Over-Discharge
Lithium-ion batteries shouldn’t go below a specific voltage. An over-discharged battery may stop working. Advanced Battery Management Systems prevent over-discharge.
o Puncture
A punctured lithium-ion battery can cause a fire. Sharp objects can damage the separator, causing a short circuit.
o Improper Disposal
Dumping lithium-ion batteries harms the environment. Responsible recycling is crucial.
o Leaks
Leaks expose the battery’s electrolyte. It’s a fire hazard. A solid-state battery eliminates this risk.
o Fast Charging
Fast charging increases the temperature. Higher temperatures can shorten the battery’s life.
o Inadequate Venting
Poor ventilation traps heat. Heat can cause the battery to fail. A well-designed venting system helps to keep the temperature in check.
o Lithium Plating
When a battery charges too fast, lithium forms metal layers. These layers affect the battery’s performance. A slower charge rate can prevent lithium plating.
o Impurities
Even small impurities can harm lithium-ion batteries. Careful manufacturing helps to maintain the quality of the components.
Measures to ensure stability
o Thermal Management
Cooling helps. Active cooling uses fans. Passive cooling uses clever design. Both aim to control temperature. Smart thermal management prolongs battery lifespan. It’s all about keeping temperatures stable. Temperature management brings safety.
o Overcharge Protection
A full battery should stop charging. Overcharge protection makes sure of that. This tech stops charging at full. It prevents battery damage. Every lithium-ion battery needs this.
o Over-Discharge Protection
Draining a battery fully harms it. Over-discharge protection stops this. It cuts power before full drain. This safeguard extends battery life. Always have over-discharge protection.
o Quality Construction
Good materials make a better battery. Solid casing protects internal parts. Secure connections keep power flowing. Quality construction ensures longer battery life. Remember, better materials mean better performance.
o Cell Balancing
Batteries have multiple cells. Each should carry equal load. Cell balancing ensures this. It prevents single cell overwork. Balanced cells mean longer battery life. Cell balancing is vital for battery health.
o Proper Venting
Batteries need to breathe. Vents let them do that. They release excess heat. Proper venting is crucial. A cool battery is a happy battery.
o Physical Protection
Durable casing shields from shocks. Dust-proof seals keep parts clean. Water-resistant coatings prevent moisture damage. Solid physical protection guarantees longer battery life.
o Separator Integrity
Separators prevent short circuits. Good separators mean safe batteries. Separator integrity ensures steady power flow. They are crucial for safe operation.
o Avoid Fast Charging
Fast charge strains batteries. It increases temperature. High temps can damage cells. Rapid charging often means shorter battery life. Avoid fast charging for longer battery health.
o High-quality Materials
Quality extends to the inside. High-grade metals conduct better. Quality separators prevent issues. Better materials mean more efficient power. High-quality components lead to better batteries.
o Periodic Inspection
Technicians look for wear and tear. Damaged parts get replaced. Regular inspection finds issues early. Periodic inspection ensures safety and performance.
o Electrolyte Stability
Electrolytes conduct ions. Stability is crucial. Unstable electrolytes cause problems. Stable ones ensure steady power. Good electrolyte stability is vital for performance.
o Safe Packaging
Batteries need secure homes. Robust packaging prevents harm. It shields against physical shocks. Good packaging also isolates from weather. Safe packaging equals secure lithium-ion battery components.
Comparisons with Other Battery Technologies!
§ Lead-Acid
Lead-acid batteries, known for their PB plates and sulfuric acid (H2SO4), don’t hold a candle to lithium-ion battery components. High in weight and with a poor energy density of 35-40 Wh/kg, they fall short. Lithium-ion batteries prove superior.
§ Nickel-Cadmium
NiCd batteries have positive plates of nickel hydroxide (NiOOH) and negative plates of cadmium (Cd). Yet, with their 40-60 Wh/kg energy density, they lack the stamina of lithium-ion counterparts.
§ Nickel-Metal Hydride
NiMH batteries, housing a hydrogen-absorbing alloy and NiOOH, sit at 60-120 Wh/kg energy density. Better, but lithium-ion still rules the roost.
§ Sodium-Sulfur
SoS batteries boast of high energy density of 150-250 Wh/kg, but operate at high temperatures. Li-ion batteries are safer.
§ Lithium-Sulfur
Li-S batteries, a close kin to lithium-ion, bring more energy (250-500 Wh/kg) to the table. But lithium-ion battery components show better durability.
§ Energy Density
Lithium-ion reigns with 150-265 Wh/kg energy density. Superior to many rivals, making it the preferred choice.
§ Power Density
The power density in lithium-ion, 250-670 W/kg, outshines many competitors. More power, less space!
§ Cycle Life
With lithium-ion, expect 500-1000 charge/discharge cycles. A lifespan longer than many similar technologies.
§ Charge Efficiency
Lithium-ion battery components have charge efficiency around 99%. Few technologies can compete.
§ Cost
Lithium-ion batteries may cost more upfront. But their longevity and power mean more bang for your buck in the long run.
§ Safety
All battery components must meet safety standards. Lithium-ion, with its reliable performance, fits the bill.
§ Environmental Impact
Lithium-ion batteries are recyclable. Less waste, more green. A strong point for lithium-ion.
§ Application Suitability
Lithium-ion batteries are versatile. Phones, laptops, electric cars – they power our world.
Conclusion
You’ve journeyed through the world of lithium-ion battery components. Each part, be it anode or cathode, has a unique role. They all work together to store and deliver energy. Now, you can see the hidden workings of these everyday items. Carry this knowledge forward.
Let it guide you when choosing or using batteries. Discover more about these powerhouses at buzzupbattery.com. Keep learning, stay powered.


