
When you choose batteries, knowing the differences matters. This guide dives deep into Lithium vs. Alkaline Batteries. Every section sheds light on vital battery aspects. Let’s navigate the energy-packed world of these batteries together.
The Science Behind Batteries!
How do Lithium Batteries Work?
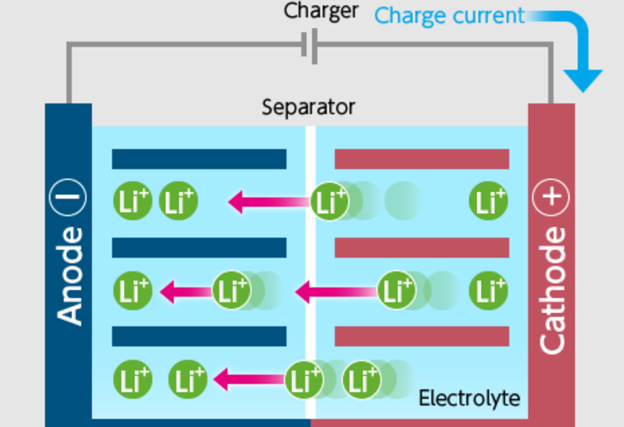
In a world of evolving power needs, understanding batteries, especially lithium vs alkaline batteries, is crucial. Both types deliver energy differently, impacting their applications.
· Electrolyte
In the battery lithium vs alkaline battle, electrolytes stand out. Alkaline batteries use an aqueous solution, while lithium ones employ organic solvents. Electrolytes permit ion movement, powering devices.
· Anode
Lithium batteries possess a lithium metal anode. Conversely, manganese dioxide serves as the anode in alkaline batteries. The anode’s role: release electrons when discharging.
· Cathode
For cathodes, lithium batteries utilize compounds like LiCoO2. Alkaline batteries use zinc powder. The cathode absorbs electrons during the discharge.
· Ions Flow
Ions movement is vital. In lithium batteries, Li+ ions flow from anode to cathode during discharge. Alkaline batteries have a similar yet chemically distinct flow.
· Discharge
During discharge, electrons flow externally from anode to cathode. Alkaline vs. lithium: the former offers steady voltage, whereas lithium provides higher energy.
· Charge Cycle
A lithium battery can endure 300-500 cycles. Alkaline batteries aren’t usually rechargeable, limiting their cycles.
· Voltage
Lithium batteries boast a voltage around 3.7V. Alkaline counterparts offer about 1.5V. Higher voltage often means more power.
· Separator
Separators in batteries keep anode and cathode apart. For lithium batteries, plastic micro-porous films are common. Alkaline batteries use a non-woven layer.
· Energy Storage
In energy storage metrics, lithium batteries excel. They store significant energy, making them ideal for high-drain devices.
· Internal Resistance
Low internal resistance ensures efficient battery performance. Lithium batteries generally have lower resistance than their alkaline counterparts.
· Chemical Reaction
Batteries work due to chemical reactions. Lithium batteries undergo intercalation, while alkaline ones involve zinc and manganese dioxide reactions.
· Electron Movement
Electron movement provides power. In lithium batteries, the movement is brisk, offering rapid power. Alkaline batteries deliver power at a steadier rate.
· Electrochemical Process
Both battery types use electrochemical processes to function. The reactions convert stored chemical energy into electrical energy, powering various devices.
How do Alkaline Batteries Work?
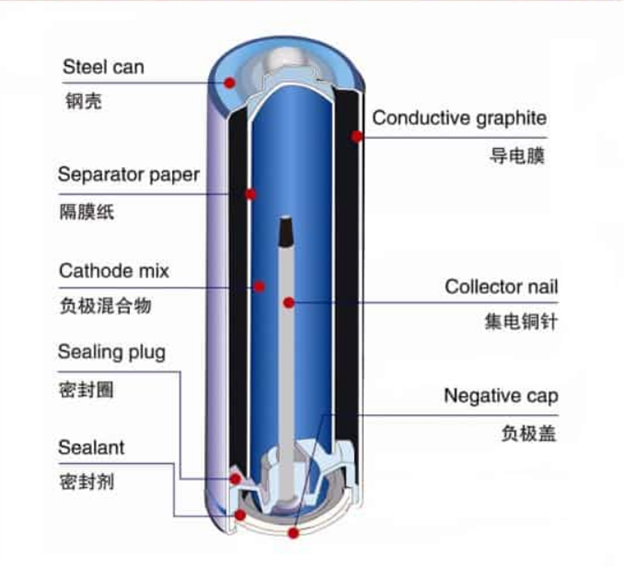
· Zinc Electrode
In a battery, the zinc electrode plays a vital role. When you compare battery lithium vs alkaline, the zinc electrode stands out in the latter. Specifically, in alkaline cells, zinc serves as the anode. Here, zinc undergoes oxidation, releasing electrons.
· Manganese Dioxide
Manganese dioxide acts as the cathode in alkaline batteries. In the alkaline vs. lithium battle, manganese dioxide’s role is unique to alkaline cells. This component accepts electrons during the discharge process.
· Electrolyte Paste
The electrolyte paste conducts ions. Alkaline batteries house an electrolyte paste which ensures effective ion movement. The transfer of ions aids in the power production.
· Oxidation
Oxidation is a process wherein atoms lose electrons. Within the battery world, especially in the context of lithium vs alkaline batteries, oxidation occurs at the anode, promoting electron release.
· Reduction
Reduction complements oxidation. At the cathode, atoms gain electrons. For alkaline batteries, manganese dioxide undergoes reduction, receiving electrons.
· Electron Flow
Electrons move from anode to cathode. Electron flow between electrodes determines the current. The faster the flow, the higher the battery’s output.
· Chemical Reaction
Batteries generate power through chemical reactions. In the realm of alkaline vs. lithium, each battery type hosts distinct reactions, with specific compounds interacting differently.
· Zinc Anode
In alkaline batteries, the zinc anode undergoes oxidation. Its role is pivotal for producing electric current by releasing electrons during this process.
· Alkaline Electrolyte
Alkaline electrolyte enhances conductivity. Its presence in alkaline batteries ensures efficient ion transfer, which in turn boosts battery performance.
· Energy Release
Batteries store chemical energy. During use, chemical reactions trigger an energy release, converting stored energy into electrical energy, powering devices.
· Voltage
Voltage, measured in volts (V), signifies electric potential. The voltage difference in electrodes influences the current flow, and in the debate of battery lithium vs alkaline, voltage remains a vital parameter.
· Cell Reaction
Inside a battery, cell reactions occur. These reactions, specific to the battery type, determine the energy output and lifespan.
· Discharge
Batteries discharge over time. Discharge signifies the conversion of chemical energy into electric energy. Monitoring discharge rates can indicate battery health.
· Battery Drain
Prolonged usage causes battery drain. As chemical reactions continue, energy depletes, and over time, battery efficiency decreases. Proper storage and usage can minimize rapid drain.
The Core Chemistry Of Lithium vs. Alkaline!
· Lithium Metal
In the lithium ion vs alkaline battery arena, lithium stands dominant. Boasting an atomic number of 3, lithium serves as the lightest metal. Next, they have a high energy density. So, for applications demanding power, professionals opt for lithium. Lastly, consider durability. The typical lithium battery surpasses 1,200 cycles. Clearly, longevity remains unrivaled.
· Manganese Oxide
Delving deeper, manganese oxide acts as a pivotal cathode material. Found predominantly in alkaline batteries, manganese oxide offers distinct advantages.
Furthermore, when combined with zinc, MnO2 (manganese oxide’s chemical formula) renders high-capacity outcomes. Notably, alkaline batteries, housing manganese oxide, present consistent voltage levels.
· Electrochemical Reactions
Comprehending the batteries’ operation necessitates grasping electrochemical reactions. Central to battery functioning, such reactions involve electron transfer.
Lithium batteries, for instance, engage in LiCoO2 reactions. Alternatively, alkaline counterparts harness the Zn/MnO2 reaction. Both processes determine battery longevity and power output.
· Alkaline Solution
An integral part of the lithium vs alkaline aa discourse centers on alkaline solutions. Predominantly, KOH (Potassium Hydroxide) forms the alkaline solution.
When in batteries, this solution facilitates smooth ion transport. Furthermore, KOH ensures that the battery doesn’t quickly drain.
· Ions Movement
Batteries, essentially, operate through ion movement. Once activated, the ions journey from anode to cathode. During their transit, energy gets released.
To illustrate, lithium batteries see Li+ ions mobilizing. In contrast, alkaline batteries witness the motion of OH- ions. Such distinctions directly impact performance and usability metrics.
· Electrode Materials
Electrode materials form the heart of any battery. For lithium cells, carbon acts as the anode, and lithium cobalt oxide as the cathode. Alkaline batteries, however, employ zinc and manganese dioxide. Both sets of materials influence energy storage, discharge rates, and overall efficiency. Recognizing such nuances enables informed choices in power solutions.
· Redox Reactions
Lithium batteries utilize Li+ ions in redox reactions. Conversely, alkaline batteries employ KOH. During discharge, lithium sheds electrons. Alkaline does similarly with manganese dioxide.
Electron flow is vital for power. Both batteries show distinct electron exchange processes. Understand that parameters like voltage result from these reactions. Both display uniqueness in electron transfer processes.
· Chemical Bonds
Chemical bonds dictate a battery’s capacity. Lithium forms strong bonds, offering extended life. Alkaline batteries, on the other hand, have weaker zinc bonds. Bond strength influences energy storage. Lithium provides longer energy storage due to firmer bonds.
· Energy States
In the energy realm, lithium batteries stand out. Higher energy states enable better performance. Alkaline batteries have lower energy peaks. An alkaline battery’s lifespan is shorter. Notably, energy states play roles in performance variation.
· Electron Configurations
Electron configurations are central in battery design. Lithium’s electron setup contributes to higher voltages. Alkaline batteries display different electron patterns, affecting their voltage output. Such configurations influence the efficiency and capacity. In essence, electron arrangements determine energy release and storage.
· Chemical Stability
Chemical stability affects battery safety. Lithium batteries, under specific conditions, might be less stable. Alkaline batteries generally have enhanced stability. Risk levels relate directly to a battery’s stability. Users benefit from knowing these stability metrics for safety reasons.
· Reaction Rates
Reaction rates indicate battery responsiveness. Lithium often exhibits faster reactions than alkaline. Rapid reaction rates enhance device performance. In devices requiring quick power bursts, lithium stands superior. Knowledge of these rates assists in battery selection.
· Catalysts
In battery reactions, catalysts play roles. Alkaline batteries might use some, speeding reactions. Lithium doesn’t always need them. Catalyst presence or absence can affect efficiency. Recognizing catalyst involvement ensures informed battery decisions.
| Property/Terms | Lithium-Based Batteries | Alkaline-Based Batteries |
| Battery Chemistry | Lithium Metal | Manganese Oxide |
| Main Electrolyte | Electrochemical Reactions | Alkaline Solution |
| Ion Movement | Rapid | Moderate |
| Electrode Materials | Various metals (e.g., LiCoO2, LiMn2O4) | Zinc and Manganese dioxide |
| Key Reaction Type | Redox Reactions | Redox Reactions |
| Stability | Chemical Stability varies (often high) | Generally stable |
| Energy Considerations | High Energy States | Moderate Energy States |
| Electron Configuration | Specific to Li-ion | Specific to Zn and MnO2 |
| Reaction Rates | Fast (depends on catalyst) | Slower than Lithium-based |
| Catalysts Used | Varied, to boost performance | Rarely used |
| Chemical Bonds | Strong (metallic, covalent) | Mainly ionic |
Table on The Core Chemistry Of Lithium vs. Alkaline!
Voltage Differences Of Lithium vs. Alkaline!
· Nominal Voltage
When examining lithium vs alkaline aa batteries, nominal voltage stands out. Typically, an alkaline battery offers 1.5V. However, a standard lithium battery provides 3V. Lithium batteries generally double the output. The difference arises due to distinct electrochemical reactions in each.
· Peak Voltage
Lithium batteries experience higher peak voltage. Shortly after activation, alkaline units might peak at 1.6V. In contrast, lithium counterparts could surge to 3.3V. The peak indicates the maximum potential energy deliverable at the outset.
· Discharge Rate
Lithium batteries boast a low self-discharge rate. Conversely, alkaline batteries tend to deplete energy when unused. To put in figures, an alkaline might lose 2-3% monthly, while lithium remains mostly undiminished.
· Load Impact
Under heavy loads, lithium batteries outperform. Their voltage remains steady, ensuring devices operate efficiently. In a similar scenario, alkaline batteries might experience a sharp decline, compromising the appliance’s functionality.
· Voltage Drop
Over time, all batteries exhibit voltage drops. Alkaline batteries, when half-depleted, could see a drop to 1.0V or lower. On the other hand, lithium batteries maintain close to their initial 3V for a significant duration before dropping.
· Chemical Dependency
Chemistry is crucial in the lithium vs. alkaline batteries debate. Alkaline batteries rely on zinc and manganese dioxide. Conversely, lithium batteries utilize compounds like LiFePO4. Such chemicals dictate performance, longevity, and energy retention.
· Cell Configuration
The internal structure or configuration impacts voltage. Lithium batteries, with their high energy density, often adopt a spiral cell configuration. Alkaline batteries, with cylindrical designs, might not harness energy as efficiently due to their layout.
· Terminal Potential
Comparing lithium or alkaline, lithium batteries show a terminal potential of about 3.0V. Conversely, alkaline batteries often display around 1.5V. The increased voltage in lithium means higher energy output. Devices requiring more power often can lean towards lithium. Battery-operated devices benefit greatly from the increased voltage.
· Cutoff Voltage
The cutoff voltage for lithium batteries typically falls around 2.5V. On the other hand, alkaline batteries’ cutoff voltage rests close to 0.9V. Once batteries reach their cutoff, they need replacement. A higher cutoff voltage means earlier replacement.
· Under-load Voltage
During operation, under-load voltage is crucial. Lithium batteries maintain a consistent voltage, offering steady performance. However, alkaline batteries may show voltage dips under heavy load, leading to inconsistent outputs. Devices might suffer from this inconsistency.
· Stability
Lithium batteries excel in stability. Extreme temperatures affect them less compared to alkaline. In contrast, alkaline batteries might leak or rupture in adverse conditions. Stable voltage in varied environments means lithium is often more reliable.
· Internal Factors
The chemical makeup defines a battery’s efficiency. For lithium batteries, the internal chemistry allows for long shelf life. Alkaline batteries, having different components, might not last as long in storage. Efficient chemical reactions influence battery lifespan.
· External Influence
Both battery types respond to external factors like humidity. Lithium batteries, however, resist moisture better. In contrast, alkaline batteries can degrade faster in humid conditions. Proper storage becomes vital to extend battery life.
· Energy Conversion
Converting chemical energy to electrical energy is the battery’s role. Lithium batteries have a higher energy density. In a lithium battery or alkaline comparison, lithium often provides more energy per unit of weight.
Energy Density Comparison Of Lithium vs. Alkaline!
· Wh/kg Measure
The Wh/kg measure refers to watt-hours per kilogram. In lithium versus alkaline batteries, the former possesses a higher Wh/kg. For every kilogram of a lithium battery, more energy gets stored. On the other hand, alkaline batteries store less energy per kilogram. Such facts establish lithium as the winner in terms of energy density.
· Volume Metric
Volume metric showcases energy storage in relation to size. Lithium batteries shine here, boasting superior energy storage in a compact form.
Conversely, alkaline batteries, though reliable, occupy more space for the same energy. Making the choice between lithium versus alkaline battery depends on the desired size-to-energy ratio.
· Capacity Ratio
Capacity ratio measures how much energy a battery can hold. While both battery types are commendable, lithium stands out. The capacity ratio for lithium is often higher, meaning longer usage periods. Yet, in environments requiring consistent, moderate energy, alkaline might suffice.
· Energy Release
Energy release defines how fast batteries give out their stored energy. Lithium batteries often release energy faster. Such a trait is crucial for devices demanding quick power bursts. Alkaline batteries, though steady, might lag in rapid energy discharge scenarios.
· Chemical Potential
Chemical potential looks into the energy from chemical reactions. Lithium batteries derive energy from robust chemical reactions, granting them an edge. Alkaline batteries have milder reactions, translating to a slightly reduced energy output.
· Power-to-weight
Power-to-weight assesses the power given concerning a battery’s weight. Lithium batteries, being lighter, offer remarkable power for their weight. In contrast, alkaline batteries might be weightier with a comparatively lower power output.
· Gravimetric Density
Gravimetric density delves into energy relative to weight. For every gram, lithium provides more energy, making them ideal for weight-sensitive applications. Alkaline batteries, while sturdy, present a lesser energy-to-weight ratio, proving lithium’s superiority in the lithium vs alkaline debate.
· Volumetric Density
When discussing lithium ion vs alkaline battery in terms of volumetric density, lithium-ion (Li-ion) stands out. Li-ion batteries possess a volumetric energy density of around 250 Wh/L.
In contrast, alkaline batteries register a lower energy density of about 80 Wh/L. Consequently, for the same volume, Li-ion cells store more energy than alkaline equivalents. Such variance influences battery selection for high-performance devices.
· Peak Performance
Alkaline batteries offer stable discharge but have limitations under high-drain scenarios. Li-ion cells, however, deliver consistent output even during high-demand tasks.
Devices needing rapid bursts of power prefer Li-ion due to its 3.7V nominal voltage; whereas alkaline’s nominal voltage sits at 1.5V. Your gadgets might perform better with a higher voltage.
· Energy Reservoir
Longevity in energy storage favors Li-ion. Over time, alkaline cells can deplete even when not in use. Li-ion’s self-discharge rate is approximately 1.5% per month, markedly better than the 2-3% of alkaline.
· Charge Efficiency
Here’s where Li-ion truly shines. Unlike alkaline batteries, which are primarily single-use, Li-ion cells can undergo numerous recharge cycles. A standard Li-ion cell can be recharged around 300 to 500 times. Alkaline rechargeables exist but pale in efficiency, often limited to a mere 50 recharges.
· Density Limit
Both battery types have caps on how much energy they can store per volume. Currently, Li-ion reaches up to 700 Wh/L in advanced models. Alkaline, despite improvements, lags behind with a maximum of approximately 120 Wh/L. Such disparities impact how long your device runs on a single charge.
· Packing Efficiency
Battery structure impacts performance. Li-ion cells utilize layered electrodes and electrolyte solutions, optimizing energy storage. Alkaline batteries rely on a paste, leading to less efficient packing. Structurally, Li-ion’s design supports better energy per unit.
· Energy Storage
Considering longer durations, which is better alkaline or lithium batteries? Li-ion is again the answer. Thanks to its superior chemical stability, it retains energy more efficiently over time. Whether stored in a drawer or powering infrequently used devices, Li-ion remains the trusted choice for extended energy retention.
Battery Lifespan Of Lithium vs. Alkaline!
· Cycle Count
Lithium batteries have about 1000-2000 cycles. In contrast, alkaline batteries average 400-800 cycles. Consequently, lithium often outlasts alkaline. Repeated usage showcases lithium’s superior endurance, affirming why lithium batteries are better than alkaline.
· Shelf-life
Store the lithium for 10-15 years without significant power loss. Alkaline? Only 5-7 years. Longer shelf-life means lithium remains powerful, even after prolonged storage. That’s advantageous for users who stock batteries.
· Discharge Patterns
Alkaline batteries lose power steadily. Lithium, however, offers consistent voltage until almost depleted. Devices powered by lithium remain consistently functional longer.
· Self-discharge
Annually, lithium batteries self-discharge 2-3%. Alkaline ones? About 0.3-2% per month. That means lithium retains its charge better when not in use, resulting in more extended reliability.
· Calendar Life
Lithium batteries hold optimal performance for 10-12 years, while alkaline spans 6-8 years. More years signify that lithium’s superiority extends over time.
· Load Cycles
Devices demanding high loads benefit from lithium. With a 3.0-3.7V typical voltage, they outperform alkaline’s 1.5V, enabling better functionality in high-drain devices.
· Depth of Discharge
Frequent deep discharges degrade alkaline. Lithium handles 80-90% DoD, proving more flexible and durable in various applications.
· Charge Frequency
Lithium excels in low-to-mid charge frequencies, retaining capacity efficiently. Such versatility enhances battery utility in both occasional and regular use devices.
· Storage Conditions
Both types prefer cool, dry storage. Yet, lithium endures extreme temperatures better, with a range of -40°C to 60°C, broadening storage options for users.
· Chemical Aging
Over time, all batteries degrade. But lithium’s Li-ion components resist chemical aging more effectively than alkaline components, assuring longer service life.
· Capacity Fade
Continuous use leads to capacity fade. Ultimate lithium vs alkaline demonstrates that lithium suffers less reduction, maintaining near-original capacity even after prolonged use.
· Cycle Degradation
Every recharge cycle slightly degrades capacity. Alkaline exhibits more degradation, leading to shorter effective lifespan compared to lithium.
· Usage Impact
Heavy usage affects alkaline more. Lithium batteries, due to their chemistry, resist wear and tear better, making them ideal for demanding devices.
· Environmental Effects
Disposal concerns? Lithium is more eco-friendly. Lesser hazardous components ensure that post-use, the environmental impact remains minimized.
Performance in Extreme Temperatures Of Lithium vs. Alkaline!

· Cold Resistance
Lithium batteries outperform under cold conditions. At -20°C, lithium provides 1.7 V output, while alkaline drops to below 1.1 V. For frigid climates, lithium is superior. Use devices safely in frosty settings with lithium.
· Heat Sensitivity
Alkaline batteries deteriorate swiftly above 60°C. Conversely, lithium tolerates up to 85°C. Electronic devices in hot surroundings benefit from lithium’s resilience. Consider climate before choosing a battery type.
· Temperature Range
Lithium operates from -40°C to 85°C. Alkaline’s range is narrower, from -20°C to 60°C. For broader temperature demands, lithium remains unmatched. Broad temperature adaptability means longer device lifespans.
· Thermal Runaway
Lithium batteries carry risks. Excessive heat might lead to thermal runaway, causing fires. Proper design and safety mechanisms become crucial. Always store and utilize batteries in recommended conditions.
· Cold Discharge
Discharge rates vary in cold environments. Lithium batteries can discharge 85% of their capacity at -20°C, whereas alkaline struggles around 65%. In chilling scenarios, lithium emerges as more reliable.
· Optimal Temperatures
The sweet spot lies between 20°C to 25°C for both types. However, lithium retains more capacity across temperature shifts. Devices remain powered longer, regardless of the surroundings.
· Chemical Stability
Alkaline batteries face leakage risks over time. However, lithium batteries boast superior chemical stability, minimizing leak potential. Proper storage mitigates risks for both types.
· Thermal Impact
High temperatures impact battery chemistry. Lithium batteries might degrade faster, impacting device longevity. Alkaline is affected too, but the decline is more predictable. Always assess temperature effects before battery integration.
· Degradation Rate
Over time, all batteries degrade. But lithium’s rate is slower than alkaline, especially in varying temperatures. Longevity is a clear advantage of lithium over alkaline.
· Operation Limits
Pushing beyond operation limits can be hazardous. Alkaline batteries might leak or rupture. Lithium batteries, while more tolerant, might overheat or catch fire. Safety guidelines remain paramount.
· Temperature Fluctuation
Rapid temperature changes affect battery performance. Lithium batteries adapt better than alkaline, ensuring consistent output. Devices operate smoothly even with rapid climate shifts.
· Heat Dissipation
Both battery types emit heat during discharge. Lithium manages better heat dissipation, ensuring safer operations. Ensuring ventilation during use can aid in safety.
· Cold Storage
Alkaline batteries can be stored in cool places without significant capacity loss. Lithium benefits from cold storage as well, prolonging shelf life. Cold storage prolongs the life of unused batteries.
· Thermal Conductivity
Conductivity defines how heat spreads. Lithium batteries generally have better conductivity, distributing heat evenly. A uniform heat distribution ensures safer, more efficient battery usage.
Weight Differences Of Lithium vs. Alkaline!
· Grams per Cell
In Lithium batteries, each cell weighs approximately 1.6 grams. In contrast, Alkaline cells typically weigh 24 grams. Clearly, a significant difference emerges. Devices requiring multiple batteries gain noticeable weight advantages with lithium.
· Weight-to-power
Every gram in a Lithium battery produces more energy. Alkaline batteries, however, need more weight to generate similar energy. Why are lithium batteries better than alkaline? Superior energy per gram is one decisive reason.
· Material Density
Lithium material has higher density than its alkaline counterpart. Consequently, Lithium offers more energy in a similar space. On the other hand, Alkaline uses larger amounts of manganese dioxide and zinc.
· Housing Weight
The casing of Alkaline batteries is often heavier. Lithium batteries use a lighter metal housing. The distinction in housing significantly contributes to their overall weight disparity.
· Electrode Mass
Lithium batteries have thinner electrodes than Alkaline. Yet, Lithium’s electrodes deliver more power. Reduced electrode weight without power compromise is another Lithium advantage.
· Anode/Cathode Ratio
The anode/cathode ratio in Lithium is optimized for longer energy delivery. Alkaline’s ratio, however, isn’t as efficient, leading to quicker energy depletion.
· Lightweight Design
Manufacturers prioritize Lithium’s compact, lightweight construction. Such an approach aids in maintaining high energy levels while cutting down excess weight.
· Mass Impact
As devices evolve, they demand efficient energy sources. Lithium, with its low mass and high output, fits the bill better than its Alkaline counterpart.
· Heaviness Factor
While Alkaline often feels heavier, Lithium provides a lightweight alternative. The former’s heaviness stems from added materials and design differences.
· Component Weight
Internal components in Lithium batteries are meticulously designed. Every piece aims for minimal weight yet high energy delivery. Alkaline components, though effective, don’t always match up in weight efficiency.
· Structural Integrity
Despite being lighter, Lithium exhibits robust structural integrity. Alkaline structures, though sturdy, don’t always offer the same weight-to-strength ratio.
· Battery Size
Though both types come in common sizes like AA or AAA, Lithium often delivers more power per unit size. Do lithium batteries last longer than alkaline? Size and energy density make the case.
· Weight Efficiency
Every gram in Lithium works harder. It delivers more energy, ensuring devices run longer and perform better.
· Device Impact
For users, Lithium’s reduced weight offers a tangible benefit. Devices feel lighter, more manageable, and often perform tasks with increased efficiency.
Battery Discharge Curves Of Lithium vs. Alkaline!
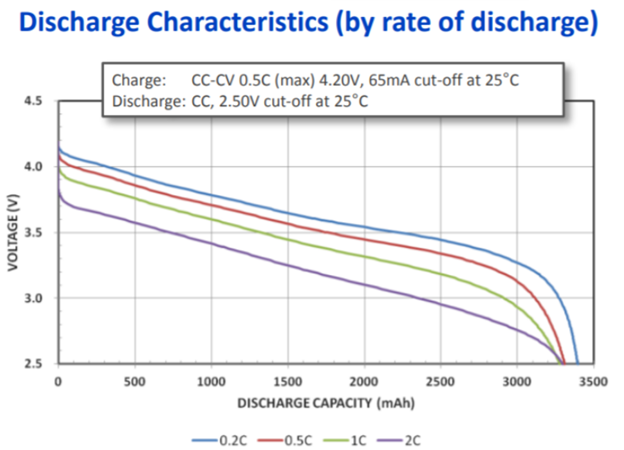
· Linear Discharge
In battery tech, linear discharge denotes consistent power release. Notably, lithium batteries offer smoother curves than alkaline counterparts. Opt for lithium for steady energy. Alkaline batteries exhibit steeper drops.
· Capacity Decline
Over time, all batteries face capacity decline. In terms of lithium vs alkaline aa battery life, lithium generally surpasses alkaline. Alkaline batteries experience swift capacity drops, reducing performance.
· Voltage Plateau
Lithium batteries boast a stable voltage plateau. Compared to alkaline, their voltage remains unchanged for longer. Such stability ensures devices function without hitches.
· End-of-life Indicator
Devices hint when power ebbs. For alkaline, the voltage drops signal depletion. With lithium, however, voltage remains constant, then drops swiftly near depletion.
· Steady Discharge
Steady discharge means consistent power output. Lithium champions in this aspect, offering reliable performance. Alkaline might fluctuate more during operation.
· Discharge Slope
Evaluating the discharge slope aids in predicting battery longevity. Steeper slopes in alkaline signify swift depletion, whereas lithium provides gradual and predictable drain.
· Rate of Drain
Devices with high energy needs, like digital cameras, drain batteries fast. Lithium batteries endure rapid drains better than their alkaline counterparts, ensuring longer use.
· Load Variability
Depending on device energy demands, load variability changes. While lithium excels under varied loads, alkaline may falter with frequent shifts.
· Curve Flattening
As energy diminishes, lithium batteries maintain a near-flat discharge curve. In contrast, alkaline curves dip, signaling a nearing end-of-life.
· Voltage Dip
Alkaline batteries might showcase minor voltage dips during use. Lithium batteries resist such dips, offering a smoother energy output.
· High-drain Behavior
Devices that pull much energy display high-drain behavior. Lithium, due to superior chemistry, typically handles such drains better than alkaline.
· Low-drain Behavior
In devices like remote controls, exhibiting low-drain behavior, lithium batteries often last years, outclassing the months offered by alkaline.
· Constant Current
Devices needing steady power benefit from a constant current. Lithium batteries ensure such a current is maintained, unlike the inconsistent flow of alkaline.
· Variable Resistance
As a battery’s state changes, variable resistance can occur. While lithium manages such changes effectively, alkaline may struggle, impacting device performance.
Shelf Life Of Lithium vs. Alkaline!
· Expiry Date
Alkaline batteries have a life of 5-7 years. Lithium counterparts last up to 10-15 years. Ensure batteries have clear expiration dates before buying.
· Storage Degradation
Storing batteries in drawers can affect their longevity. Lithium often resists degradation better than alkaline.
· Self-discharge Rate
Over time, all batteries lose charge. Lithium batteries tend to have a lower self-discharge rate than alkaline.
· Packaging Impact
The packaging of batteries plays a role in preserving quality. Vacuum-sealed packs are optimal for long-term storage.
· Humidity Effects
Dry environments are best for batteries. High humidity can negatively impact both lithium and alkaline.
· Capacity Retention
Over time, batteries lose their capacity. Lithium batteries retain capacity better than alkaline in most cases.
· Chemical Stability
Chemical reactions within batteries affect their performance. Lithium tends to be more chemically stable.
· Longevity Factors
Lithium often lasts longer in devices. High-drain devices benefit most from lithium.
· Moisture Impact
Wet conditions can damage batteries. Always ensure dry storage to maximize battery lifespan.
· Optimal Conditions
Best conditions include cool, dry places away from direct sunlight. Proper storage ensures battery efficiency.
· Seal Integrity
Check for intact seals on batteries. Broken seals can lead to battery leakage, especially in alkaline.
· Environmental Impact
Dispose of batteries responsibly. Incorrect disposal can harm the environment. Recycling centers accept both types.
Internal Resistance and Efficiency Of Lithium vs. Alkaline!
· Ohmic Value
The ohmic value, measured in ohms (Ω), marks a difference. In comparison, Lithium batteries generally present lower internal resistance than their Alkaline counterparts. Lower resistance in Lithium means efficient energy flow. Devices then perform longer and stronger.
· Charge Transfer
Lithium batteries often excel in charge transfer efficiency. Efficient charge movement is due to fewer obstructions in Lithium. Energy gets utilized more effectively than in Alkaline.
· Polarization
Polarization concerns unwanted accumulations, hindering battery efficiency. Alkaline batteries tend to suffer more from polarization. Lithium, however, manages better, ensuring consistent power.
· Conductivity Impact
Electrical conductivity in Lithium batteries generally surpasses that of Alkaline. Greater conductivity means faster energy transfer. Devices can work more reliably.
· Current Flow
Current flow indicates how energy moves. Lithium typically ensures a steadier current flow. As a result, devices often experience fewer energy disruptions.
· Resistance Increase
Over time, batteries show increased resistance. Alkaline batteries often display a swifter rise. On the other hand, Lithium remains steadier, promising longevity.
· Chemical Buildup
Chemical residues can impede battery function. Alkaline types might show more buildup over time. Lithium batteries manage to reduce such chemical obstacles.
· Aging Effects
Aging can affect battery performance. Alkaline batteries might decline faster. Yet, Lithium batteries often prove more resilient to the effects of time.
· Energy Losses
Energy loss can be a concern. Lithium batteries, however, ensure minimal losses. They typically offer greater energy conservation than Alkaline.
· Heat Generation
Heat is a byproduct of energy use. Alkaline batteries might produce more heat. Lithium batteries, in contrast, manage to stay cooler, promoting safety.
· Resistance Measurement
Measuring resistance offers insights into efficiency. Instruments like the multimeter aid in gauging this. Often, Lithium batteries showcase superior metrics.
· Efficiency Metrics
Efficiency metrics evaluate battery performance. Various tests reveal that Lithium batteries often outperform Alkaline in multiple efficiency areas.
· Power Delivery
Regarding power delivery, Lithium batteries reign superior. Their design ensures a consistent and robust power output, suitable for high-demand devices.
· Flow Restrictions
Flow restrictions can impede energy movement. While Alkaline batteries might exhibit more restrictions, Lithium usually ensures an unhindered flow.
High-drain Performance Of Lithium vs. Alkaline!
· High Current
In a comparative study, lithium batteries show higher mA. Alkaline counterparts offer less. Lithium vs alkaline aa battery life varies. Moreover, devices with high current demands prefer lithium for optimum functioning. Remember, the selection greatly influences device output.
· Rapid Discharge
Rapid discharge rates favor lithium. Alkaline batteries tend to deplete faster under high discharge. In scenarios demanding quick energy releases, lithium stands out as the superior choice.
· Voltage Maintenance
Over time, a battery’s voltage might dip. Lithium batteries maintain voltage better than alkaline. Voltage stability ensures consistent device operations.
· Heavy Load
Devices with heavy load needs consume more energy. In these cases, lithium batteries outpace alkaline ones. Alkaline batteries might struggle with hefty power demands, resulting in performance drops.
· Power Surge
During power surges, devices require more energy. Lithium batteries cater to these spikes efficiently. Meanwhile, alkaline batteries might falter, not meeting the increased demand swiftly.
· Device Demand
Each device has unique energy demands. Whether are alkaline and lithium batteries interchangeable depends on that demand. For high-demand gadgets, lithium often emerges as the preferable option.
· Performance Drop
Alkaline batteries face a significant performance drop in cold temperatures. On the contrary, lithium batteries thrive, offering consistent performance even in chilly conditions.
· Capacity Requirements
Capacity denotes how much power a battery can store. Between lithium vs alkaline batteries life, lithium boasts a higher capacity, ensuring longer usage periods before replacements become necessary.
· Endurance Levels
Durability matters. In endurance tests, lithium batteries consistently surpass alkaline, proving their capability to withstand rigorous usage patterns.
· Continuous Drain
For devices needing continuous power, lithium excels. Alkaline batteries might deplete quicker under continuous drain scenarios, making lithium a superior option for uninterrupted use.
· Intermittent Drain
Intermittent power demands can be tricky. While both battery types can handle it, lithium usually exhibits a better performance curve, ensuring device longevity.
· Peak Performance
In terms of peak performance, lithium batteries often shine brighter. Their ability to deliver high power when needed makes them a favored choice for many.
· High-demand Devices
Devices like digital cameras or GPS units require more power. Here, lithium batteries, due to their higher energy density, offer a clear advantage over alkaline counterparts.
· Efficiency Retention
Over time, efficiency retention becomes paramount. Between lithium vs alkaline batteries life, lithium batteries tend to retain their efficiency better, ensuring sustained device performance.
Battery Size Variants Of Lithium vs. Alkaline!
· AA Size
Notably, AA lithium batteries often yield higher energy than alkaline ones. Voltages for lithium hover around 3.6V, whereas alkaline outputs 1.5V.
· AAA Size
AAA lithium and alkaline batteries differ in capacity. Alkaline batteries usually offer 1200mAh, while lithium can reach up to 3000mAh. Longer-lasting devices prefer the latter for obvious reasons.
· Button Cells
Small yet vital, button cells in watches or calculators use both types. Lithium cells typically have a longer lifespan and better lithium vs alkaline discharge curve.
· C Cell
Devices needing consistent power favor C-cell lithium over alkaline. Even under extensive use, lithium showcases efficient performance due to its inherent design.
· D Cell
Flashlights, radios, and toys utilize D cells. Alkaline vs lithium batteries environment concerns arise, with lithium being less harmful upon disposal.
· Coin Types
Lightweight devices, especially medical equipment, choose coin-type lithium for reliability. Alkaline counterparts, though cheaper, might not deliver the same durability.
· Prismatic
Prismatic lithium batteries serve mobile phones and laptops proficiently. Their flat structure aids in optimal space use, setting them apart from cylindrical alkaline batteries.
· Pouch Format
Pouch format batteries, found in smartphones, mostly adopt lithium due to superior energy density. Their slim nature facilitates design flexibility, an edge over alkaline.
· Cylindrical
Power tools and cameras benefit from cylindrical lithium batteries. With superior lithium vs alkaline batteries voltage, such devices operate longer and more efficiently.
· Custom Shapes
Unique devices demand custom-shaped batteries. Lithium, due to versatile fabrication, fits perfectly, whereas alkaline might not always measure up.
· Device-specific
Some gadgets require device-specific batteries. Often, manufacturers lean towards lithium for consistency, long life, and better energy output.
· Thickness Variance
Lithium batteries, with varied thickness, cater to ultra-slim devices. In contrast, alkaline batteries have fixed sizes, limiting adaptability.
· Diameter Differences
Devices with tight diameter specifications favor lithium. Its flexible design parameters accommodate devices better than rigid alkaline structures.
· Compactness
Compact lithium batteries serve miniaturized electronics efficiently. Alkaline, although dependable, might not always meet compactness requisites.
| Feature/Type | AA Size | AAA Size | Button Cells | C Cell | D Cell | Coin Types | Prismatic |
| Material | |||||||
| Lithium | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Alkaline | Yes | Yes | Yes | Yes | Yes | No | No |
| Voltage (V) | |||||||
| Lithium | 1.5 – 3 | 1.5 – 3 | 3 | 1.5 – 3 | 1.5 – 3 | 3 | 3.2 – 3.7 |
| Alkaline | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | — | — |
| Energy Density (Wh/L) | |||||||
| Lithium | 250 | 180 | 300 | 230 | 270 | 280 | 250 |
| Alkaline | 110 | 80 | 100 | 95 | 130 | — | — |
| Diameter (mm) | |||||||
| Lithium | 14.5 | 10.5 | Varies | 26.2 | 34.2 | Varies | Varies |
| Alkaline | 14.5 | 10.5 | Varies | 26.2 | 34.2 | — | — |
| Compactness | |||||||
| Lithium | High | High | Very High | Medium | Low | Very High | High |
| Alkaline | Medium | Medium | Medium | Low | Very Low | — | — |
Table on Battery Size Variants Of Lithium vs. Alkaline!
Rechargeability Factor Of Lithium vs. Alkaline!
· Charge Cycles
Lithium batteries offer approximately 1,200 charge cycles. Alkaline, on the other hand, are mainly single-use. For repeated use, lithium proves superior. Many users favor lithium for durability.
· Memory Effect
Alkaline batteries don’t suffer from memory effect. However, lithium-ion can show signs. Devices run longer when using a battery without memory effect.
· Chemical Stability
In challenging conditions, lithium shows better chemical stability. Alkaline batteries can leak, causing damage. Proper storage ensures longevity.
· Degradation Rate
Lithium batteries degrade slower. Alkaline batteries might start losing charge faster, especially in high-drain devices.
· Recharge Limit
Every battery has a recharge limit. Lithium typically surpasses 1,000 full recharges. Alkaline doesn’t support multiple recharges.
· Capacity Retention
Over time, batteries lose capacity. Lithium retains more energy, making them reliable for essential gadgets, like a lithium or alkaline smoke detector.
· Efficiency Loss
Alkaline batteries might not perform well in cold temperatures. Lithium maintains a consistent output, even in freezing weather.
· Overcharging Risk
Charging poses risks. Overcharging lithium can be hazardous. Alkaline batteries are not designed for recharging.
· Recharge Speed
Users often ask, “are lithium batteries better than alkaline?” Lithium batteries recharge faster, often within hours, giving them an edge.
· Self-discharge
All batteries self-discharge. Lithium self-discharges at about 2% monthly. Alkaline loses charge faster when stored.
· Refresh Cycles
Refreshing helps restore battery health. Lithium can benefit from periodic refresh cycles, improving performance.
· Recharge Indicators
Modern gadgets show battery levels. A clear indicator helps users know when to recharge, vital for understanding what’s better alkaline or lithium batteries.
· Charging Circuitry
Quality charging circuitry ensures safety. Lithium batteries require specialized circuitry to prevent overcharging, an essential safety measure.
· Reusable Factor
Another debate on what’s better lithium or alkaline batteries centers on reusability. Lithium is rechargeable and reusable, while alkaline is mainly disposable.
Leakage and Durability Of Lithium vs. Alkaline!

· Seal Breakage
In batteries, seals prevent leakage. Lithium batteries have robust seals. Alkaline batteries sometimes have weaker seals. Leakage occurs more in alkaline. Proper sealing ensures longer battery life. Both batteries use distinct materials for sealing.
· Electrolyte Escape
Electrolytes carry charge. In lithium, they rarely escape. But alkaline batteries might experience more escapes. Leakage from electrolytes reduces battery efficiency. Effective containment ensures optimal performance. Different containment methods apply to both batteries.
· Chemical Reactions
Lithium and alkaline batteries undergo varied chemical reactions. Chemical stability is higher in lithium. Alkaline reactions might produce gas, causing bulging. Monitoring these reactions helps in maintaining battery health.
· Corrosion
Metallic corrosion can harm batteries. Lithium batteries resist corrosion better. Alkaline batteries sometimes show signs of corrosion. Corrosion affects the battery’s conductive paths. Keeping batteries dry can minimize corrosion.
· Long-term Storage
Storing batteries affects their life. Lithium batteries hold charge better over time. Alkaline batteries can lose charge faster during storage. Proper storage conditions, like cool temperatures, benefit both.
· Environmental Stress
Batteries react to environmental changes. Lithium handles extreme temperatures better. Alkaline batteries might not perform well in cold. Environmental adaptability varies between the two.
· Material Degradation
Over time, battery materials degrade. Lithium batteries show slower degradation. Alkaline batteries might degrade faster. Material quality plays a significant role in battery lifespan.
· Internal Pressure
Pressure build-up is risky. Lithium manages internal pressures efficiently. Alkaline might face bursting risks. Devices need batteries that can handle internal pressures well.
· Bursting Risk
Batteries can burst if mistreated. Lithium batteries have a lower bursting risk. Alkaline, if overcharged, can burst. Safety mechanisms in devices prevent bursting.
· Housing Integrity
The outer shell protects battery internals. Lithium often has stronger housing. Alkaline housing might be more prone to dents. Robust housing ensures longer battery service.
· Containment Layers
Layers within batteries keep components intact. Lithium uses advanced layers for containment. Alkaline might have simpler layers. Proper layers prevent unwanted chemical mixing.
· Protective Coatings
Batteries come with protective coatings. Lithium often has superior coatings. Alkaline coatings might wear out sooner. These coatings combat external damages.
Battery efficiency drops with age. Lithium usually faces fewer age-related issues. Alkaline can see a quicker drop in performance. Regularly checking batteries helps detect age-related problems.
· Exposure Impact
Exposure to elements affects batteries. Lithium stands firm against most exposures. Alkaline might suffer in humid conditions. Ensure batteries remain in safe environments for longevity.
Safety and Hazards Of Lithium vs. Alkaline!
· Thermal Runaway
Excessive heat in batteries causes thermal runaway. For lithium batteries, this can occur at 150°C. Alkaline batteries have a lower risk. Proper design minimizes this danger. Avoid puncturing and extreme temperatures.
· Venting
Batteries release gases under stress. Lithium batteries, when faulty, may vent toxic gases. Alkaline batteries vent less often. Venting can damage devices. Always monitor battery health.
· Short Circuits
Internal or external paths causing unintended current flow define short circuits. Lithium batteries can heat rapidly during such events. Alkaline batteries remain relatively stable. Insulating terminals reduces risks.
· Swelling
Lithium batteries can bulge due to gas buildup. Alkaline batteries experience less swelling. Bulging indicates battery failure. Replace swollen batteries promptly.
· Flammable Electrolytes
Electrolytes in lithium batteries can ignite. Flames cause serious harm. Alkaline batteries have less flammable components.
· Combustion Risk
Faulty lithium batteries can combust. Alkaline batteries have a lower combustion risk. Avoid exposing batteries to high heat sources. Safe handling reduces incidents.
· Chemical Burns
Alkali leakage from alkaline batteries can harm skin. Lithium batteries also contain harmful chemicals. Handle damaged batteries with care. Wear protective gloves.
· Gas Release
Decomposing lithium batteries release hydrogen gas. Alkaline batteries emit less harmful gases. Ensure proper ventilation in storage areas.
· Handling Precautions
Lithium and alkaline batteries have difference in voltage and chemistry. Incorrect handling can lead to failure. Read lithium battery manufacturer’s guidelines before use.
· Disposal Concerns
Environmentally, improper disposal harms ecosystems. Lithium batteries require specialized disposal methods. Alkaline batteries are less hazardous but still need proper disposal.
· Leakage Issues
Alkaline batteries leak more often. Lithium batteries rarely leak, but consequences can be severe. Regularly check batteries in devices.
· Over-discharge
Depleting lithium batteries below certain voltage can damage them. Alkaline batteries tolerate over-discharge better. Monitor battery charge levels.
· Overheating
Lithium batteries can overheat under heavy load. Alkaline batteries usually remain cool. Overheating reduces battery lifespan. Keep batteries in a cool environment.
· Explosion Potential
Explosions are rare but dangerous. Lithium batteries have a higher explosion potential. Alkaline batteries have minimal risk. Ensure proper charging and handling.
Battery Capacity and mAh Ratings Of Lithium vs. Alkaline!
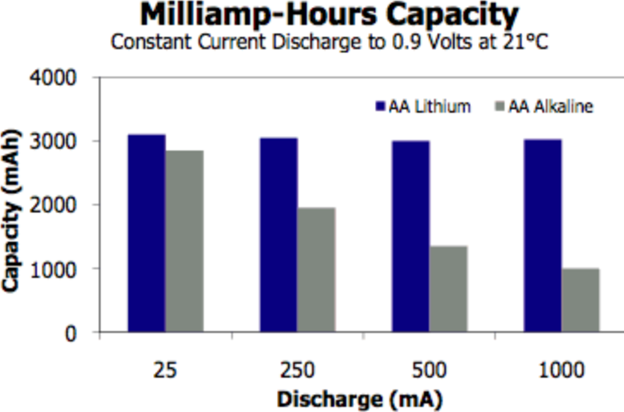
· Capacity Measures
In battery science, capacity tells how much energy a battery holds. Typically, lithium batteries possess higher capacity. Electronic devices run longer with lithium. Trust in lithium for sustained power.
· mAh Indication
Milliampere-hour (mAh) denotes battery longevity. Higher mAh ratings signify extended battery life. Generally, lithium batteries exhibit superior mAh than alkaline. So, for longer operation, consider lithium.
· Wh Ratings
Watt-hour (Wh) presents energy content. Lithium batteries tend to provide more Wh, implying more stored energy. Consequently, for demanding gadgets, lithium becomes the go-to.
· Discharge Rates
Batteries lose energy over time. Discharge rates depict this energy reduction. In performance, lithium outpaces alkaline, presenting slower discharge. Professionals prioritize lithium for stable energy flow.
· Duration Metrics
The span a battery powers a device denotes its duration. Lithium consistently scores higher. Many industries pick lithium for extended durations.
· Energy Reservoir
Every battery functions as an energy reservoir. In the duel of alkaline vs lithium batteries difference, lithium stands out. Its dense energy storage ensures robust performance.
· Total Output
Total energy a battery can provide is its output. Undoubtedly, lithium batteries give superior total output.
· Efficiency Ratios
Efficiency reflects how batteries utilize stored energy. On efficiency scales, lithium batteries often overshadow alkaline. Resultantly, many experts lean towards lithium for optimal efficiency.
· Device Compatibility
Batteries must sync with devices. Lithium batteries boast wide compatibility, suiting diverse gadgets.
· Usage Duration
Duration reflects battery’s active life. Repeatedly, lithium outperforms alkaline in longevity. Industries lean on lithium for enduring tasks.
· Load Impacts
Under heavy loads, battery behavior will change. Lithium remains consistent, whereas alkaline might falter. For heavy-duty applications, trust lithium.
· Endurance Indicators
Endurance suggests how long batteries can withstand demands. Once again, lithium dominates with superior endurance metrics.
· Standard Capacities
Set capacity benchmarks exist. Frequently, lithium surpasses these standards, while alkaline stays within norms. Professionals favor lithium for exceeding benchmarks.
· Peak Performance
At maximum demand, batteries reveal true potential. Lithium batteries often reach and maintain peak performance better than alkaline.
Interchangeability in Devices Of Lithium vs. Alkaline!
· Compatibility
Many devices use AA or AAA cells. However, are lithium and alkaline batteries the same? No, but both types fit these devices. Still, knowing battery specs aids optimal performance.
· Device Requirements
Electronics need specific energy levels. What is the difference between lithium and alkaline? Alkaline often gives 1.5V, while lithium provides 3V.
· Voltage Match
Voltage is electrical pressure. Lithium usually pushes out more. Ensuring correct voltage prevents device damage.
· Size Fit
AA and AAA are common sizes. Both lithium and alkaline batteries come in these sizes.
· Terminal Alignments
Battery terminals – positive and negative – provide energy flow. Incorrect alignment stops power delivery.
· Retrofitting
Sometimes devices designed for alkaline batteries can use lithium with adjustments. Retrofit kits help with this. Yet, research before making changes.
· Polarity Consistency
Positive and negative terminals, the battery’s polarity, must match device slots. Wrong placements can harm devices.
· Electrical Characteristics
Do lithium or alkaline batteries last longer? Lithium often outlasts alkaline, especially in high-drain devices. Evaluating battery characteristics optimizes usage.
· Safety Checks
Lithium vs alkaline batteries for smoke detectors is a debated topic. Lithium batteries, due to their longevity, are preferred. However, always perform safety checks.
· Device Instructions
Instructions guide on preferred batteries. What is better, alkaline or lithium batteries? Manuals often specify, helping users choose.
· Consumer Awareness
Being informed aids decision-making. Lithium or alkaline batteries for trail cameras, for instance, have varied opinions.
· Conversion Kits
Kits aid transition from alkaline to lithium in specific devices. However, always ensure these match device specifications.
· Special Adapters
Adapters allow devices to use different batteries. Yet, knowing battery specs remains critical.
· Manufacturer Recommendations
Companies provide battery guidelines. Are lithium and alkaline batteries interchangeable? Sometimes, but always consult recommendations.
Applications Of Lithium vs. Alkaline!
· Watches
Precision matters. Watches require consistent power. With lithium vs alkaline battery weight, lithium proves lighter. Many premium watches adopt lithium. Lighter batteries mean sleeker designs. Longevity also favors lithium. With minimal self-discharge, lithium can guarantee accurate timekeeping. Experts prefer lithium for high-end timepieces.
· Clocks
Time doesn’t wait. Wall clocks demand longevity. Alkaline batteries often serve this need. Yet, for digital clocks, lithium’s consistent voltage excels. Notably, lithium vs alkaline aaa batteries discussions highlight lithium’s longer lifespan.
· TV remotes
Quick channel swaps need reliable power. Pile lithium vs alkaline debates indicate lithium’s efficiency. Most modern remotes integrate lithium. No one enjoys frequent battery changes. Lithium ensures longer TV binge sessions.
· Smoke detectors
Smoke detectors demand uninterrupted power. The ultimate lithium vs alkaline contest has a clear winner. Lithium’s longevity ensures constant vigilance. Alkaline might wane faster, risking functionality.
· Flashlights
For brightness and duration, battery type matters. Flashlights employing lithium vs alkaline 9v battery display different power curves. Lithium ones stay bright longer. Experts suggest lithium for critical applications.
· Toys
Playtime shouldn’t end abruptly. Considering lithium vs alkaline aa battery test, lithium outlasts alkaline. Lithium ensures longer play sessions.
· Hearing aids
Clear hearing is non-negotiable. Between alkaline vs lithium rechargeable batteries, lithium holds a charge longer. Sound quality remains consistent. For those with hearing needs, lithium remains the trusted choice.
· Wireless mics
For crystal-clear sound, choose wisely. 9 volt lithium vs alkaline debates note lithium’s consistency. Live performances benefit from lithium. Distortions reduce, ensuring better sound quality.
· Gaming controllers
Game on! Gamers prefer consistent response times. Assessing which batteries are better alkaline or lithium, lithium emerges superior. Prolonged gaming sessions demand lithium-powered controllers.
· Calculators
Mathematical tasks demand undisturbed power. For prolonged use, lithium offers better reliability. Engineers and students alike recognize lithium’s unmatched consistency.
· Car alarms
Security can’t waver. Car alarms using lithium stay vigilant longer. Uninterrupted power ensures better car safety. In the security industry, lithium is often recommended.
· Thermostats
In home thermostats, lithium and alkaline batteries difference becomes evident. Lithium batteries last longer. Constant temperature monitoring is maintained. Alkaline batteries, though cheaper, might require frequent replacements. Opt for energy efficiency.
· Pagers
During the ’90s, pagers were pivotal. Lithium ion vs alkaline determined pager efficiency. Lithium provided longer usage hours. Meanwhile, alkaline lacked such longevity. Professionals chose based on battery life.
· Laser pointers
Presentations demand uninterrupted tools. Between lithium vs alkaline aaa, lithium leads in lifespan. Alkaline might interrupt crucial moments. Reliable presentations need powerful batteries.
· Digital thermometers
Medical accuracy is non-negotiable. Lithium ion battery vs aa alkaline batteries displays significant contrasts. Lithium ensures consistent readings. Conversely, alkaline’s shorter lifespan poses risks.
· Glucose meters
Diabetic patients need precise results. Lithium ion vs alkaline battery life matters. Longer battery life means fewer replacements. Lithium offers better dependability than alkaline.
· Portable radios
Communication clarity depends on battery choice. Between cr2032 lithium vs alkaline, lithium lasts longer. For outdoor adventures, such choice makes a difference. Ensure uninterrupted signals.
· Nightlights
Children’s safety at night is paramount. Lithium vs alkaline aa battery decision impacts night-long illumination. Lithium, with extended battery life, promotes security. Alkaline may fade sooner.
· Backup systems
Emergencies necessitate reliable backups. When comparing alkaline vs lithium batteries difference, lithium proves superior. It provides longer runtimes. In crises, every second counts.
· Camping gear
Adventurers know battery importance. Gear with lithium batteries offers sustained functionality. On the other hand, alkaline might cut short your adventures. Choose wisely for wilderness survival.
· Home security
Homes demand uninterrupted protection. Home energy storage battery systems powered by lithium ensure longer surveillance. Alkaline systems, while cost-effective, might compromise safety durations. Secure residences with powerful batteries.
· Medical devices
Hospital gadgets need unfaltering power. Patient welfare depends on lithium vs alkaline batteries. Lithium supports longer operational hours. Alkaline, though common, might need frequent switches.
· Electric shavers
Morning routines demand efficiency. Shavers powered by lithium ensure consistent trims. Alkaline ones may halt midway. Grooming sessions deserve the best batteries.
· Cordless doorbells
Visitor alerts shouldn’t fail. Doorbells with lithium batteries rarely miss rings. Alkaline-powered ones might. Make homes welcoming with reliable doorbell batteries.
Conclusion
In understanding Lithium vs. Alkaline Batteries, many factors come into play. From their chemistry to their usage, each has unique features. Armed with this knowledge, making informed battery choices becomes easier. For more battery insights and products, visit BuzzupBattery. Always choose wisely.


