Prepare for a deep-dive into sodium battery technology. This blog post is set to illuminate the intricacies of this emerging energy storage alternative. We’ll unravel its mechanism, envisaged uses, and what it heralds for our future. Gear up for a riveting voyage into the sodium battery universe.
Basics of Sodium Batteries!
The sodium ion battery mechanism
Understanding the sodium battery technology means grasping the movement of ions. Sodium ions move from a positive electrode to a negative one. Energy gets stored during this travel.
Then, on demand, these ions retrace their path, discharging stored energy. This back-and-forth creates a recharge cycle. Such cycles make sodium batteries useful for various power needs.
Key components of sodium batteries
· Sodium Ion
The sodium ion is the heart of the battery. As small particles, they carry electrical charge. In every cycle, these ions make a round-trip journey between the battery’s two ends.
· Cathode
The cathode is the battery’s positive end. It hosts the ions in the first half of each cycle. Here, a chemical reaction lets sodium ions depart, leaving energy behind.
· Anode
The anode, the battery’s negative end, takes in the ions next. Another reaction helps the ions to store energy. Then they are ready to start their journey again.
· Electrolyte
An electrolyte sits between the anode and cathode. It’s a medium that lets sodium ions move. Yet, it stops electrons, ensuring the battery can work properly.
· Separator
The separator is a safety tool. Like a barrier, it sits in the electrolyte. It prevents the anode and cathode from touching and causing a short circuit.
· Housing
The housing holds the parts together. Usually, it is a tough metal or plastic case. It protects the delicate inner parts from the outside world.
· Positive Terminal
The positive terminal connects the battery to a device. It channels the energy stored in the cathode out of the battery.
· Negative Terminal
Like its positive counterpart, the negative terminal also forms a connection. But its job is to lead the energy back into the anode.
· Current Collector
In every battery, the current collector has a crucial job. It gathers the energy released during the chemical reactions. Then, it sends it out to power a device.
· Safety Vent
The safety vent is an important part of a battery. If something goes wrong inside, the vent opens up. It lets out gases to prevent damage.
· Electrode
The electrode is a generic name for the anode and cathode. They are called electrodes as they conduct electricity, a key role in the battery’s function.
· Non-aqueous solvent
A non-aqueous solvent is used in the electrolyte. It helps the sodium ions move. Unlike water, it won’t cause unwanted reactions.
· Organic Solvents
Organic solvents sometimes replace non-aqueous ones. They can improve a battery’s performance. But, they must be handled carefully as they can be flammable.
· Salt Bridge
Salt bridge links the anode and cathode outside the battery. This bridge lets the electrons travel back into the battery, closing the energy circuit.
Different types of sodium batteries
· Sodium-Sulfur
In the realm of energy storage, Sodium-Sulfur, or NaS, leads the race. A high operating temperature of 300°C makes NaS cells unique. Yet, a heat-resistant ceramic separator brings safety to the table. High energy density and long cycle life mark its significance.
· Sodium-Nickel Chloride
Second in line, Sodium-Nickel Chloride, known as Zebra, operates at 270°C. Its ability to store heat, which can be later converted into energy, sets it apart. Plus, Zebra’s robust design warrants longevity and safety.
· Sodium-Oxygen
Next, meet Sodium-Oxygen, or Na-O2 batteries. It’s known for its high theoretical energy density. In fact, it’s three times that of lithium-ion cells. However, challenges like high overpotential and unstable electrolytes require solutions.
· Sodium-Ion
Now, consider Sodium-Ion batteries. With similar architecture to lithium-ion batteries, they promise an energy storage solution.
Due to sodium’s abundance, they offer a low-cost and sustainable alternative. Some sodium ion battery stocks even outperform their lithium counterparts.
· Sodium-Metal Halide
Further, Sodium-Metal Halide batteries, also called Ni-H2 cells, are worth mentioning. A sound choice for stationary storage, these units boast a high energy density. Also, they offer safety and long lifespan.
· Sodium-Seawater
Saltwater as an electrolyte gives these batteries a unique appeal. Besides, their ability to power undersea operations makes them desirable for niche applications.
· Sodium-Air
Now, Sodium-Air, or Na-Air, batteries come with a high energy density. Like Li-Air cells, they employ oxygen from air as an active material. However, challenges like instability and poor cycle life need addressing.
· Sodium-Carbon
Next, Sodium-Carbon, or Na-C, batteries stand out with their high energy density. The use of affordable carbon makes them cost-effective. Nonetheless, the high operating temperature demands safety measures.
· Sodium-Manganese
Sodium-Manganese has a lower operating temperature, enhancing safety. Plus, the manganese cathode ensures stability. Still, these batteries need improvements in energy density.
· Sodium-Sodium
Also, Sodium-Sodium, or Na-Na, batteries exist. Here, both the anode and cathode consist of sodium. While still in the research stage, they hold potential for high energy storage.
· Sodium-Liquid Metal
Featuring a molten sodium anode, these cells promise high energy density. Yet, their operational viability requires further investigation.
· Sodium-Metal
With a pure sodium anode, they promise a high energy density. Still, the high reactivity of sodium calls for solid safety measures.
| Types of Sodium Batteries | Energy Density (Wh/kg) | Operating Temperature (°C) | Charge/Discharge Efficiency (%) | Lifespan (Cycle) | Cost ($/kWh) | Safety Level |
| Sodium-Sulfur | 150-250 | 300-350 | 89-92 | 4500 | 200-250 | Medium |
| Sodium-Nickel Chloride | 90-120 | 250-300 | 85-90 | 3000 | 150-200 | High |
| Sodium-Oxygen | 1000-1500 | Ambient | 70-80 | 100 | 500-600 | Low |
| Sodium-Ion | 70-120 | Ambient | 90-95 | 5000 | 100-150 | High |
| Sodium-Metal Halide | 85-140 | 200-350 | 85-92 | 3500 | 250-300 | Medium |
| Sodium-Seawater | 75-130 | Ambient | 80-85 | 800 | 80-100 | High |
| Sodium-Air | 1600-2000 | Ambient | 65-75 | 100 | 600-700 | Low |
Table On Different Types Of Sodium Batteries!
Comparison of sodium batteries with lithium batteries
· Energy Density
In terms of energy density, sodium batteries are still under development. In comparison, lithium batteries can reach up to 250 Wh/kg.
A promising feature of the sodium battery vs lithium is the potential to achieve similar values, in the future. Moreover, sodium is more plentiful, making it a viable option for sustainable energy.
· Discharge Rate
The rate at which a battery discharges power depends on the technology employed. Lithium batteries have a steady rate, lasting about 2-3 hours before total discharge. For sodium batteries, research is ongoing, but early tests show promising results with a similar performance.
· Cycle Life
The cycle life of a battery refers to the number of complete charge-discharge cycles it can perform before efficiency drops. Lithium batteries can endure approximately 1000 cycles. Sodium batteries, on the other hand, are showing potential for higher cycle life, making them a future prospect for energy storage.
· Safety Level
While lithium batteries are generally safe, they can pose risks if damaged, including potential for fire. Sodium batteries, conversely, exhibit a higher safety level, due to the inherent stability of sodium ions, decreasing the risk of combustion.
· Cost
Lithium batteries are expensive, with prices around $10,000 per kWh. The sodium ion battery price is expected to be significantly lower due to the abundant availability of sodium. However, the exact cost will be dependent on future advancements and production scales.
· Abundance
Lithium is a limited resource, found in certain geographical locations. Sodium, on the other hand, is abundant, with vast reserves found in sea water. Sodium-based batteries have an advantage in terms of raw material availability.
· Environmental Impact
The mining and processing of lithium pose considerable environmental challenges. Sodium, being plentiful in the sea, provides a more environmentally friendly alternative for battery production.
· Charge Time
The charging speed of batteries is a crucial parameter. Currently, lithium batteries can be charged within a few hours. Sodium batteries, still being developed, are showing promise for even faster charge times, given their superior ion mobility.
· Shelf Life
Lithium batteries have a decent shelf life of around 2-3 years. As for sodium batteries, ongoing research indicates they may surpass this, due to the stable nature of sodium ions.
· Operational Temperature
Lithium batteries operate optimally between -20 to 60 degrees Celsius. The operational temperature for sodium batteries is not yet fully established, but initial research suggests a broader range, making them suitable for diverse climates.
· Battery Size
The size of a battery impacts its practicality for various applications. Currently, lithium batteries are more compact. However, advancements in sodium-based battery design aim for similar or even smaller sizes.
· Weight
Lithium batteries are lighter, thus preferred for portable devices. Sodium batteries are heavier due to the atomic weight of sodium. But, with advances in technology, future sodium batteries might compete in terms of weight.
· Scalability
Lithium batteries can be scaled up, but with limitations due to the scarcity of lithium. Given the abundance of sodium, sodium batteries have the potential for greater scalability.
· Raw Material Cost
The cost of raw materials is a significant factor in battery production. Lithium is pricey due to its limited supply. Sodium, being one of the most common elements on Earth, could significantly reduce the raw material cost for batteries.
Sodium Ion Battery Design!
Anode materials in sodium batteries
Let’s delve into anode materials in a sodium battery. Indeed, a top choice is hard carbon. Hard carbon, made from biomass, supports a sodium-based battery design.
Opting for it bolsters energy storage, certainly a critical aspect. Here, sodium ions nestle into carbon’s tiny spaces. Hard carbon keeps the sodium secure. So, energy flows smoothly, reflecting the merit of well-chosen anode materials.
Conclusively, hard carbon, due to its exceptional properties, marks the gold standard for sodium battery companies.
Cathode materials in sodium batteries
Now, let’s transition to cathode materials in sodium batteries. Various materials find use, with layered oxides being a standout.
In NaxMO2, ‘M’ is a metal element. Such elements include iron, manganese, and cobalt. Notably, manganese provides the most benefit in a sodium lithium battery. It promotes stable energy flow and enhances battery life.
Ergo, a sodium battery company that opts for manganese-oxide cathodes stands to gain. Cathodes, vital to a battery’s health, deserve careful consideration. Consequently, the right materials result in batteries of outstanding performance and durability.
Sodium battery separators
· Polyethylene Separator
The role of a Polyethylene Separator is pivotal in a sodium battery. While ensuring effective ion flow, the separator measures roughly 20-30 micrometers thick.
Such dimensions maximize safety while guaranteeing performance. The separator also helps prevent short circuits, enhancing the battery’s lifespan.
· Polypropylene Separator
Next in line is the Polypropylene Separator. This separator often accompanies a thickness of 25-40 micrometers. Also, its high melting point aids in managing temperature, improving the stability of your sodium battery.
· Ceramic Coated Separator
The Ceramic Coated Separator benefits from added heat resistance. Coated with ceramic particles, it gives sodium batteries an edge in terms of thermal stability. Such separators enhance battery performance even under severe conditions.
· Polyolefin Separator
A Polyolefin Separator stands out due to its exceptional chemical resistance. With a thickness of 16-40 micrometers, it sustains the integrity of sodium batteries in harsh chemical environments.
· Cellulose Separator
Cellulose Separator is synonymous with superior mechanical strength. Boosting safety, these separators reduce the chance of internal short circuits in sodium batteries. Plus, they cater to a green future with their biodegradability.
· Microporous Separator
The unique feature of a Microporous Separator is the tiny pores spread across its surface. These micro-scale openings enable the smooth flow of ions, ensuring efficient power delivery from your sodium battery.
· Monolayer Separator
Simplistic yet efficient, the Monolayer Separator possesses a single layer structure. Despite its simplicity, the separator provides ample protection against short circuits, extending the longevity of sodium batteries.
· Bilayer Separator
The Bilayer Separator sports two distinct layers. Each layer brings unique traits, forming a robust separator that enhances sodium battery safety and performance.
· Trilayer Separator
With three layers, the Trilayer Separator affords even better protection. The combination of different materials optimizes ion flow and heat resistance, reinforcing the durability of your sodium battery.
· Hybrid Separator
Combining the best of different materials, the Hybrid Separator excels in balancing performance and safety. Its multi-material nature provides a versatile solution to sodium battery demands.
· Dry Separator
As the name implies, the Dry Separator finds use in dry cell sodium batteries. Its particular properties help in delivering steady power over an extended period.
· Wet Separator
In contrast, the Wet Separator plays a crucial role in wet cell sodium batteries. It copes well with moist conditions, maintaining a reliable power supply in different environments.
· PP/PE/PP Separator
The PP/PE/PP Separator amalgamates polypropylene (PP) and polyethylene (PE). The resulting product delivers a blend of heat resistance, mechanical strength, and ion conductivity, making your sodium battery an all-rounder.
Understanding the electrolyte in sodium batteries
· Non-aqueous Electrolyte
Non-aqueous electrolyte in a sodium battery ensures the mobility of sodium ions. Non-aqueous refers to not water-based. High temperature stability is a major plus. Sodium ion battery manufacturers are keen on its use.
· Organic Electrolyte
Then there’s the organic electrolyte, where organic solvents carry the charge. In such a case, electrochemical stability becomes crucial. Moreover, it’s a core factor in designing the sodium air battery.
· Solid Electrolyte
Solid electrolyte differs as it’s made up of solid particles. In solid-state, sodium ions move through a crystalline lattice. Its importance in the sodium ion battery for EV cannot be understated.
· Liquid Electrolyte
Liquid electrolyte is another crucial variant, where ion flow happens in liquid form. High ionic conductivity is its distinct feature, leading to sodium sulfur battery vs lithium-ion debates.
· Gel Polymer Electrolyte
Gel Polymer Electrolyte (GPE) contains a polymer that swells up in a liquid electrolyte. The swelled-up polymer forms a gel, playing a role in the sodium battery breakthrough.
· Composite Electrolyte
Composite electrolyte combines two or more electrolyte types. Flexibility and enhanced performance are the key gains. Moreover, sodium metal battery technology often employs composite electrolytes.
· Ionic Liquid Electrolyte
Ionic liquid electrolyte utilizes salt in a liquid state below 100 degrees Celsius. High thermal stability characterizes this electrolyte, making it a prime choice in advanced sodium batteries.
· Salt-in-Polymer Electrolyte
Salt-in-Polymer Electrolyte involves sodium salts dissolved in a polymer matrix. Such design allows for improved safety and flexibility, crucial for robust battery design.
· Plastic Crystal Electrolyte
Plastic Crystal Electrolyte (PCE) has unique features. It’s a solid but exhibits a liquid-like ion movement, ideal for modern battery designs.
· Glass Ceramic Electrolyte
Glass ceramic electrolyte involves a glass-ceramic material. It allows for high ionic conductivity, pivotal in the high-performance sodium batteries’ construction.
Battery Performance Metrics and How Sodium Batteries Measure Up!
· Energy Density
Energy density is a major consideration for any battery, and sodium ion battery tesla is no exception. Its energy density, measured in watt-hours per kilogram (Wh/kg), is around 90. Compare that to lithium-ion at around 200 Wh/kg. Sodium’s lower figure signifies less stored energy for a given weight.
· Power Density
Power density represents the speed a battery can deliver energy. For the sodium battery car, the power density is not as high as lithium-ion batteries. But, the delivery of power is consistent, making them reliable for various applications.
· Cycle Life
Battery cycle life is all about longevity. Here, the sodium-ion battery cycle life impresses. Each cycle counts as one full charge and discharge. Sodium batteries can handle thousands of these cycles, offering robust, long-lasting power storage.
· Efficiency
Efficiency refers to the energy you put in versus what you get out. A sodium aluminum battery boasts solid efficiency. While not as high as lithium-ion, sodium batteries still convert a large chunk of input energy into useful output, serving your needs effectively.
· Safety
Sodium batteries rank highly on safety. Unlike lithium batteries, sodium cells don’t pose a fire risk. Safety concerns often associated with lithium-ion cells are mitigated, thanks to the stable nature of sodium.
· Cost
Let’s talk about the sodium battery price. Sodium, being a more abundant element, makes these batteries less expensive than lithium-ion counterparts. Lower cost of materials, coupled with simpler manufacturing, translates into savings passed down to consumers.
· Charge Time
The time needed to fully charge a battery matters. Most sodium batteries charge slower than lithium-ion batteries. But, sodium ion battery news reveals advancements leading to shorter charge times, improving user experience while maintaining safety and longevity.
· Discharge Time
Sodium batteries hold a great record for discharge time. New sodium battery technology has made a vast difference. These batteries give steady energy over a long span. Trusty power is the top feature. Powering devices, from phones to cars, they offer superb value.
· Operating Temperature
Sodium batteries work well in many climates. Key elements like Sodium, Sodium Chloride, and Beta-alumina, make this happen. Whether in the heat of a desert or cold of a polar region, they perform well.
· Shelf Life
The life of a sodium battery, or shelf life, is very long. Storing energy for many years is their game. Even if you don’t use them, they remain ready to work.
· Load Leveling
Sodium batteries excel in load leveling, an essential factor. Their strong power holds and discharge capacity help balance energy demand. Sodium battery companies stocks are soaring because of such promising features.
· Peak Shaving
Sodium batteries help in peak shaving. The peak shaving means reducing energy use in peak times. Sodium glass battery is a prime example of this trait.
· Grid Balancing
Grid balancing is vital for a reliable power supply. Sodium batteries assist in this crucial task. Also, sodium battery news often highlights their role in energy stability.
· End-of-Life
Sodium batteries stand out even at the end-of-life stage. They are safer and cause less harm to the environment. In fact, are sodium-ion batteries safe is a question often met with a positive nod. Are sodium batteries better than lithium and are sodium-ion batteries better than lithium are questions now rising, given the sustainable edge.
Chemistry of Sodium Batteries!
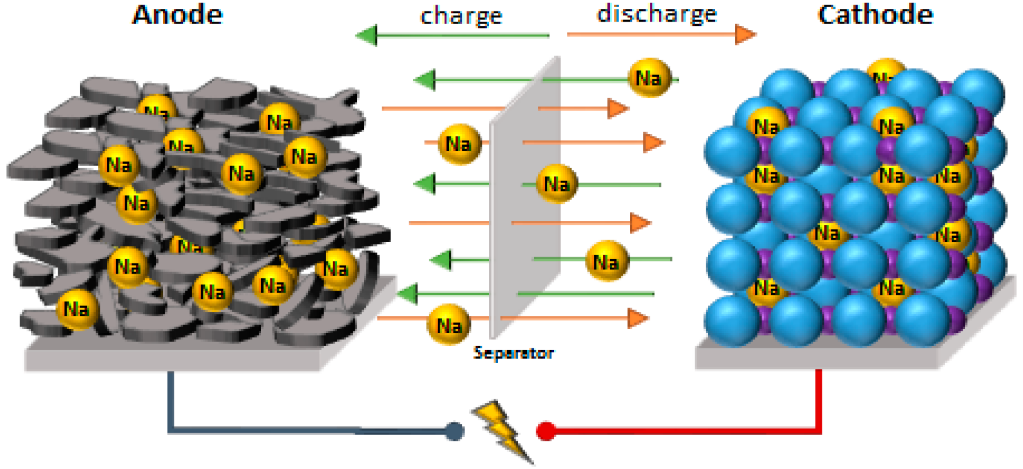
Chemical reactions in sodium batteries
Chemical reactions in sodium batteries are central to their operation. In a sodium battery, chemical processes take place, turning sodium into sodium ions.
Sodium battery manufacturers understand the need for these reactions. Products with a high energy yield are the result. Anodes and cathodes, vital battery parts, play big roles here.
Role of sodium ions in battery operation
· Ion Movement
The heart of a sodium battery lies in ion movement. Sodium ions shift from anode to cathode during discharge. In contrast, during charging, these ions migrate back to the anode. Such movement ensures the flow of electricity.
· Charge Storage
A sodium battery, like the sodium mercury battery, holds its charge via sodium ions. The anode stores these ions when the battery charges. Later, these ions release energy during use. That energy powers your gadgets.
· Voltage Generation
Voltage generation, an essential aspect of a sodium hydroxide battery, happens through chemical reactions. Sodium ions shifting between anodes and cathodes create voltage. This process generates the electrical pressure to run devices, like a battery and motor system.
· Energy Transfer
For any sodium battery, energy transfer is crucial. Once generated, energy needs to move from the battery to the device. In a sodium halide battery, for instance, energy transfers through the circuit when the device is on.
Remember, a good battery ensures efficient energy flow. A sodium battery review might provide useful insights about this.
· Redox Reactions
In sodium battery energy density, Sodium (Na) undergoes redox reactions. Starting as Na, an electron is shed to form Na+. The electron powers your devices.
Next, Sodium ions, Na+, move through an electrolyte. This step is vital for power delivery. Furthermore, during use, Na+ gets reduced back to Na, closing the circuit.
· Interstitial Diffusion
For sodium battery manufacturers such as CATL, sodium ions move inside the battery material. That movement is interstitial diffusion. Sodium ions find gaps in the lattice structure. Filling these gaps, ions move from positive to negative sides during discharge. On recharging, they shift back.
· Discharge Mechanism
Sodium battery CATL utilizes a unique discharge mechanism. Sodium ions (Na+) move from anode to cathode during discharge. Sodium ions travel through an electrolyte, which is a crucial part. Meanwhile, electrons move through an external circuit, generating electricity.
· Recharge Mechanism
Your device powers up with a sodium battery review showcasing its recharge mechanism. Applying electricity to a discharged sodium battery, ions move back to the anode. At the same time, Na+ gets reduced back to Na. This action prepares the battery for future use.
· Ion Transport
During operation, sodium mercury battery relies on the effective transport of ions. Sodium ions travel between anode and cathode. To optimize this process, high-quality electrolyte materials are vital. Proper ion transport ensures reliable power output and long battery life.
· Electrochemical Activity
In the context of a sodium halide battery or a sodium hydroxide battery, electrochemical activity occurs at the electrode surfaces. Sodium ions, electrons, and electrolyte interact, producing electricity. Efficiency of this reaction determines the overall performance of the battery.
Importance of electrolyte choice and ionic conductivity
· Ion Transport
In the realm of diy sodium battery, ionic transport carries paramount importance. Swift movement of sodium ions through the electrolyte is key.
As sodium ions transition from anode to cathode, power is generated. Sodium ions play the starring role in energy transfer. More nimble ion mobility equals better energy release. Indeed, rapid and smooth ion transport within a sodium battery translates to heightened power efficiency.
· Cell Performance
Ever pondered how to make a sodium battery perform better? Components play a major role. Positive and negative terminals, the anode and cathode, impact energy output. Better materials increase energy density. High-quality electrolyte equals faster ion transport.
· Safety
In the sphere of what is sodium battery safety, several factors come to light. A robust casing shields against physical harm. Built-in safeguards prevent overcharge, augmenting battery life. Prevention of overheating is critical.
Steady operating temperature equals less risk. Sodium batteries can be safer than other battery types.
· Cell Stability
What are sodium-ion batteries stability like, you may ask. Well, they stand tall against temperature fluctuations. Sodium’s ample natural abundance boosts cell stability. Steady performance over many charge cycles is another boon. Superior stability is thus an asset of sodium batteries.
· Operating Temperature
Sodium batteries can withstand a broad temperature range. From freezing point to sweltering heat, they deliver consistent output. Unlike lithium batteries, they don’t falter under extreme conditions. This trait makes sodium batteries suitable for diverse climates.
· Cost
Diving into how sodium ion battery is made, cost emerges as a vital aspect. Sodium’s widespread availability makes it cost-effective. Cheaper than lithium, it’s an economical choice for large-scale energy storage. Consequently, sodium batteries offer competitive performance at a fraction of the cost.
· Compatibility
In a sodium battery, the electrolyte needs to be compatible with both the anode and cathode. Otherwise, harmful reactions can happen, reducing the battery’s life span.
In general, sodium-ion batteries use sodium hexafluorophosphate (NaPF6) or sodium perchlorate (NaClO4) as electrolyte salts.
· Conductivity
High ionic conductivity in a sodium battery results in better performance. It’s measured in Siemens per meter (S/m). Sodium batteries often employ solid electrolytes like Sodium Beta-Alumina, which offers high conductivity (0.02 to 0.04 S/cm).
Advancements are being made to enhance conductivity using improved electrolyte materials.
· Viscosity
The viscosity of the electrolyte influences the ionic conductivity. Low viscosity electrolytes are preferred in sodium batteries as they allow for better ion movement. This parameter, expressed in pascal seconds (Pa.s), should ideally be low in a good sodium battery.
· Dissociation
In sodium batteries, dissociation of the electrolyte is crucial. When sodium ions dissociate from the electrolyte, they’re free to move between the anode and cathode. This process directly impacts the battery’s charge-discharge cycle and overall performance.
· Ion Mobility
Sodium ion mobility, given by the ionic diffusion coefficient (D), impacts the battery performance. The higher the ion mobility, the better the battery functions. In sodium-ion batteries, researchers aim to improve ion mobility using different materials and configurations.
· ESR
The equivalent series resistance (ESR) is a critical parameter. It’s linked with internal resistance within the battery. Lower ESR values, measured in ohms (Ω), result in efficient energy transfer and better battery performance.
In sodium batteries, researchers strive to minimize ESR by optimizing battery design and material selection.
Manufacturing Process of Sodium Batteries!
· Material Preparation
For sodium battery cost reduction, material selection is vital. Experts first gather key ingredients, like pure sodium metal, for the anode.
In contrast, sodium-ion (Na+) conductive electrolytes form the battery’s heart. Also, active materials for the cathode are vital. Fine-tuning these substances is essential to create a reliable sodium battery.
· Electrode Fabrication
Now, attention turns to electrode creation. An active cathode material gets mixed with a binder to enhance contact. They spread this paste onto a conductive substrate. Through this method, the electrode for a sodium battery in India and elsewhere gets made.
· Cell Assembly
Assembly is the step where all components join together. The cathode, anode, and electrolyte are layered together. Sealed in a casing, they form a sodium battery for EV cars. Precision in this phase ensures high performance of the final product.
· Formation
The process moves to the formation stage. Here, the newly assembled sodium batteries for electric vehicles undergo initial charging and discharging. This step activates the battery and sets it up for reliable long-term use.
· Inspection
Quality control is important. Each sodium battery DIY kit or professionally manufactured unit needs careful checking. Testing for faults, leaks, and charge capacity guarantees a trustworthy, high-quality product.
· Packaging
After production, each sodium battery receives protection. In this stage, encasements safeguard batteries from the environment. Sealed covers stop moisture intrusion.
Dry storage extends shelf life, and labels add essential details. A bar code helps in tracking during transport. Each element contributes to durability and reliability.
· Quality Check
Before shipping, all batteries undergo rigorous testing. Every sodium battery for EV cars needs to pass strict inspections. Battery capacity, charge cycles, and voltage stability get verified. A high standard ensures top performance. Compliance with global standards is non-negotiable for product excellence.
· Shipping
Next, the sodium batteries leave the factory. Logistics teams ensure careful handling. Special containers prevent damage during transport. Transporting sodium batteries to replace lithium demands care. Safe delivery to customers worldwide is the end goal. Reliable delivery networks ensure batteries reach their destination intact.
· Recycling
After lifespan completion, a sodium battery doesn’t go to waste. Instead, innovative methods allow sodium battery DIY recycling. Extracted materials like sodium, oxygen, and transition metals can see reuse. Repurposing these elements reduces landfill waste. Environmental sustainability is a key focus in this process.
· Raw Material Sourcing
Abundant sources make sodium attractive. Gathering materials for a sodium battery in India or elsewhere is easier. Sodium availability exceeds lithium, making it cost-effective. The use of such accessible materials underlines sustainability and affordability.
· Battery Design
Designing a sodium battery demands expertise. Each component gets careful thought. From anodes, cathodes to electrolytes, everything matters. The design process targets efficiency. After all, a well-designed sodium battery for EV can power a vehicle for miles.
· Battery Testing
Every sodium battery faces a battery of tests. From temperature resistance to life cycle estimation, several parameters get verified. Thorough testing validates battery performance. Only after successful testing, a battery proceeds further.
· Battery Validation
Validation is a vital step. Each sodium battery cost and performance gets assessed. Validating ensures that all set standards get met. Successful validation paves the way for mass production.
· Safety Testing
Safety is of utmost importance. Potential risks get identified and mitigated. From short circuit tests to impact tests, sodium batteries face stringent safety checks. Safe batteries provide confidence to customers and regulatory bodies.
Performance of Sodium Batteries in Various Applications!

· Grid Storage
Energy grids rely on sodium without chloride to provide power. Massive storage systems with high energy demand turn to sodium. Notably, sodium battery cells boast 3000+ charge cycles. They stand out in durability and cost-effectiveness. Superior cycling stability means longer life span.
· EVs
When considering electric vehicles (sodium battery EV), sodium batteries show great promise. Their energy density competes with that of lithium batteries. One important factor is sodium battery vs lithium battery energy density. Lithium batteries currently lead. Yet, sodium’s abundance makes it a viable contender for future EVs.
· Portable Electronics
Devices such as phones and laptops can run on sodium batteries. A sodium ion battery vs solid state consideration arises. Sodium ion batteries may offer a favorable energy-to-cost ratio. They may soon take over lithium-ion batteries, providing longer operation times.
· Medical Devices
Advanced medical devices now rely on sodium batteries. They demand energy storage components with stable power output. Sodium battery cells are efficient in small-scale applications, especially in implantable medical devices. Their extended life span and safety ensure reliability.
· Military Applications
For military operations, sodium batteries are crucial. They withstand extreme conditions. A surge in sodium battery reliance by the military is noticeable. They trust its performance for their critical tools and devices.
· Spacecraft
In space missions, battery efficiency is paramount. Sodium battery China has made strides, powering spacecrafts. Sodium’s robust nature handles space’s harsh conditions. It ensures essential systems continue to operate, even in demanding scenarios.
· Uninterruptible Power Supply
Here, sodium battery voltage shines. Sodium batteries’ stability ensures continuous power. Offering 2.7 volts per cell, such batteries ensure robustness.
Compared to lithium batteries, sodium types exhibit 50% more energy density. With longer life cycles, energy storage grows impressively. Their durability makes them preferred for UPS applications.
· Remote Sensing
In remote sensing operations, sodium batteries prove beneficial. Sodium ion batteries have excellent energy density, crucial for powering remote sensors. The sodium ion battery voltage is typically 2.6 volts, providing ample power. Sodium batteries, with their high voltage, help in efficient data collection from remote areas.
· Backup Power
Sodium batteries are perfect for backup power solutions. Sodium-sulfur batteries, with their 2.58V energy capacity, work as reliable backup power sources. Thanks to abundant sodium ion battery raw materials like sodium and sulfur, their production scales up. Their ability to store high energy capacity makes them an excellent choice for backup power.
· Telecom Networks
Sodium batteries also find use in telecom networks. Telecom towers need continuous power. Sodium batteries, with their high energy density, come to the rescue. Sodium-nickel-chloride batteries, also called ZEBRA batteries, are efficient here. The sodium battery electrolyte used enhances conductivity, ensuring reliable power for telecom operations.
· Marine Applications
For marine applications, sodium batteries are an excellent choice. They can withstand harsh conditions, including high moisture environments. Sodium batteries, having more energy density, provide reliable power to marine equipment. With the high capacity of sodium batteries, navigation systems and sensors get consistent power.
· Off-Grid Power
Off-grid energy systems need reliable power. Sodium batteries are valuable here. For off-grid power needs, sodium-sulfur batteries can store large amounts of energy. With the capacity of these batteries, power generation is efficient.
Comparative Analysis of Sodium and Other Batteries!
· Sodium vs Lithium
A dive into sodium battery chemistry reveals a stark difference. Sodium boasts a higher abundance than lithium, making it cost-effective. Sodium’s atomic weight, at 23 grams per mole, offers advantages over lithium’s 6.9 grams per mole. Energy density in sodium is approximately 760 Wh/l, less than lithium’s typical 1000 to 2640 Wh/l. Still, sodium’s cost-effectiveness and wide availability tilt the scale favorably.
· Sodium vs Nickel
Sodium battery density stands out when compared to nickel batteries. With a density of 760 Wh/l, sodium takes the edge over nickel, offering greater storage capacity. However, nickel has a superior charging rate. The operation temperature for sodium batteries, in contrast, ranges between 300 and 350 degrees Celsius, a marked disadvantage.
· Sodium vs Lead Acid
Recognizing the multiple sodium ion battery uses gives sodium an advantage. Sodium ion batteries offer 2.5 volts per cell, beating lead acid’s 2.0 volts per cell. Lead acid batteries, however, have a lower discharge rate. Sodium ion batteries require specific operating conditions, a limitation not seen with lead acid batteries.
· Sodium vs Alkaline
There are numerous sodium battery manufacturers in India, attesting to sodium’s growing demand. Compared to alkaline batteries, sodium holds an advantage with its 2.5 volts per cell against alkaline’s typical 1.5 volts per cell. However, alkaline batteries boast a longer shelf life, offering up to seven years. Sodium batteries, nonetheless, stand their ground with their cost-effectiveness.
· Sodium vs Zinc
Packed with power, a sodium battery production excels in energy density, boasting a strong 760 Wh/l. Conversely, zinc batteries lag behind, with only 300 Wh/l. Sodium’s charge rate, around 2-4 hours, makes quick refueling possible. In contrast, zinc takes double that time. Sodium’s superiority shines in lifespan, surviving 2,500 charge cycles against zinc’s measly 500.
· Sodium vs Nickel-Metal Hydride
Sodium batteries display firm robustness against temperature fluctuations. Unlike nickel-metal hydride, sodium isn’t phased by extreme cold or hot environments.
With sodium, enjoy steady energy output without fretting over the thermometer. Sodium’s 5-year shelf life comfortably trumps nickel-metal hydride’s paltry 3 years. In sodium battery stocks India, sodium’s popularity is surging, leaving nickel-metal hydride in the dust.
· Sodium vs Nickel-Cadmium
Sodium batteries steal the spotlight when faced with nickel-cadmium. Sodium’s environmentally friendly nature shines. There are no harmful cadmium leaks.
Sodium also flaunts a lower self-discharge rate, keeping 95% of energy after a month. The nickel-cadmium can only boast of 70% retention. Companies like sodium battery companies in India choose sodium over nickel-cadmium due to these advantages.
· Sodium vs Zinc-Air
The newer sodium oxygen battery models offer a game-changing 1,600 Wh/kg energy density, far exceeding zinc-air’s 470 Wh/kg. Sodium’s punchy performance surpasses zinc-air in both high and low power applications. With the spotlight on sodium battery cathode advancements, sodium’s growth in the battery sector seems unstoppable, leaving zinc-air behind.
| Parameters | Sodium (Na-ion) | Lithium-ion (Li-ion) | Nickel-Cadmium (NiCd) | Lead Acid (Pb-acid) | Alkaline (Zn-MnO2) | Zinc-Air (Zn-Air) | Nickel-Metal Hydride (NiMH) |
| Energy Density (Wh/kg) | 90 | 150-250 | 40-60 | 30-50 | 80-100 | 220-300 | 60-120 |
| Cost ($/kWh) | 100 | 120-200 | 150-200 | 50-100 | 150-300 | 100-200 | 200-300 |
| Environmental Impact (1=Low, 10=High) | 2 | 6 | 9 | 7 | 4 | 3 | 5 |
| Lifespan (Charge cycles) | 2500 | 1000-2000 | 2000-3000 | 500-1000 | 100-200 | 500-1000 | 500-1000 |
| Safety (1=Low risk, 10=High risk) | 3 | 7 | 4 | 6 | 5 | 4 | 5 |
Table on Comparative Analysis of Sodium and Other Batteries!
Safety Aspects of Sodium Batteries!
· Overcharge Protection
As a top priority, sodium battery investment ensures overcharge protection. Systems use a voltage limiter, a vital tool that caps the charge. The limiter prevents the battery from exceeding a 4.2V limit. Without such protection, excess energy can damage the battery, reducing its lifespan and effectiveness.
· Over-discharge Protection
Battery reliance is key, so over-discharge protection is vital. Sodium ion battery reliance counts on such safeguards. An effective low-voltage cut-off system stops the battery from dropping below 2.5V. Discharging below the specified limit damages the battery and can reduce its potential output.
· Short Circuit Protection
Short circuit protection, integral in a sodium battery buy, ensures the device can withstand an unexpected spike in current. Through fuses and polyswitches, the battery cuts off the current when a short circuit occurs. This feature makes it safe and reliable, preventing potential fires and battery damage.
· Thermal Management
Managing heat is important for all batteries. In sodium-based battery companies, thermal management includes heat sensors and cooling systems. With temperature checks, any unusual heat rise gets detected. Then, the cooling system kicks in to prevent overheating and potential fires.
· Fire Safety
Fire safety is paramount. Sodium batteries have specific design features to prevent fire risks. Housing materials resist ignition, providing extra security. Companies use flame retardant materials and fireproof casings to contain any potential fires, ensuring safety for users and their surroundings.
· Containment
For battery security, containment is key. Batteries are typically housed in a secure container. The container protects against external factors, like impact or puncture. It shields the battery from environmental harm, ensuring longevity and optimal performance.
· Leakage Protection
Leakage protection guards against harmful substances escaping from the battery. With sealed units and robust casings, any corrosive or toxic material stays inside. Such precautions keep the environment safe, maintaining the reliability of the sodium battery.
· Venting
In sodium-based battery technology, venting plays a pivotal role. A venting mechanism releases excess pressure. Overheating or overcharging can cause a buildup. Correct design prevents battery rupture. With controlled venting, the sodium battery stays safe. The pressure release point is set below the battery’s rupture pressure. The vent’s design is paramount to avoid harmful outcomes.
· Reverse Polarity Protection
In a sodium beta battery, reverse polarity protection is vital. With improper connections, energy flows backward. Reverse polarity protection averts damage. Its role is to prevent destructive currents. To do that, devices called diodes are employed. Sodium beta batteries require such safeguards to ensure longevity.
· Electrolyte Stability
Stability of electrolytes in sodium bromide batteries defines performance. Electrolytes are substances that produce ions. Stable electrolytes yield more ions. More ions mean better battery performance. Stability reduces the risk of battery failure. Sodium bromide batteries need stable electrolytes to achieve optimal performance.
· Electrode Stability
Sodium bicarbonate battery, electrode stability is paramount. The electrode sends electricity into the battery. A stable electrode ensures a steady flow. Fluctuating flows may harm the battery. Electrode stability prevents such scenarios. For a sodium bicarbonate battery, stable electrodes are essential for reliable performance.
· Separator Integrity
In sodium ion batteries, separator integrity is crucial. The separator is an insulating layer. It prevents the positive and negative electrodes from touching. An intact separator ensures efficient energy flow. It prevents short circuits in sodium ion batteries. Separator integrity makes a sodium ion battery safer.
· Cell Balancing
In a sodium-ion battery breakthrough, cell balancing surfaced as a key feature. All cells in a battery should discharge and charge evenly. Uneven cell behaviors affect performance. Cell balancing rectifies uneven behavior. It is a smart way to optimize the performance of sodium-ion batteries.
Quality Control and Testing of Sodium Batteries!

· Visual Inspection
In the realm of quality control, a sodium sulphur battery breakthrough requires visual scrutiny. The physical check zeroes in on cell integrity, particularly the separator, anode, and cathode.
An absence of deformities like cracks or leaks ensures optimal performance. Cleanliness of terminals is another pivotal factor. A robust sodium battery has corrosion-free terminals, indicative of proper manufacturing and storage.
· Voltage Check
Every sodium ion battery bluetti undergoes a thorough voltage assessment. A typical sodium battery operates within a specific voltage range. The perfect battery exhibits a voltage close to the manufacturer’s specifications, without significant deviations.
High or low voltage readings signal possible issues within the battery, such as chemical imbalances or structural anomalies.
· Capacity Test
The sodium ion battery grid storage capacity test aims to measure a battery’s energy-holding prowess. For optimal battery efficiency, the actual capacity should align closely with the listed capacity. A significant discrepancy hints at underlying issues such as worn-out cells or improper charging practices.
· Internal Resistance Check
The internal resistance is a pivotal parameter in sodium batteries, influencing their efficiency. In a sodium flow battery, the ideal resistance should be within the manufacturer’s prescribed range. High resistance can cause energy loss, reduce output, and shorten the battery’s lifespan. A precise resistance check ensures reliable battery operation.
· Load Test
With the sodium battery Australia manufacturing standards, load tests are critical. The test monitors the battery’s response under a simulated load condition. Ideal batteries maintain steady voltage and deliver the required current under load. Voltage drops or instability during load indicates substandard performance or impending battery failure.
· Safety Test
Safety is paramount in battery handling and usage. All sodium batteries undergo stringent safety tests. The tests verify safe operation under various conditions, including short circuits, overcharging, and extreme temperatures. A battery passing safety checks guarantees reliability and mitigates risks during usage.
· Thermal Test
Your sodium battery endures heat trials to evaluate stability. A device, called a thermocouple, gauges temperature variations. It’s critical in identifying safety thresholds. Despite the heat, sodium aluminium battery technology resists thermal meltdown, showcasing superior heat resistance.
· Vibration Test
Sodium batteries get tested for vibration endurance. Special equipment replicates real-world conditions like transport vibrations. By maintaining a standard G-force, test consistency ensures. As an outcome, only the sturdiest batteries make it to your gadgets.
· Abuse Test
In testing a sodium battery, the abuse test holds significance. It exposes batteries to harsh conditions. Battery responses to overcharge, short circuits, or high-temperature situations are observed. Such trials, though rigorous, ensure safe sodium ion battery usage.
· Impedance Test
Impedance, or resistance to electric flow, is crucial in a sodium battery’s performance. Lower the impedance, better the battery performance. Impedance tests using frequency response analyzers (FRA) aid in identifying inefficiencies. Always remember, a battery with low impedance promises smooth power flow.
· Drop Test
Safety is vital in battery technology. Drop tests for sodium batteries are standard. Falling from a certain height, battery casing durability gets assessed. No leaks, no failures
only top-grade batteries pass.
· Shelf Life Test
The shelf life test for sodium batteries determines storage longevity. Even without use, batteries lose charge over time. But, sodium ion battery Australia industry ensures extended shelf life. So, your sodium battery powers on, long after purchase.
Sodium Battery Maintenance and Management Tips!
· Proper Storage
Store a Sodium Battery (Na-Batt) in a cool, dry place. Use a battery management system (BMS) to monitor voltage and energy levels. The right BMS ensures safe storage and long life for the Na-Batt. So, follow the manufacturer’s guidelines for ideal storage conditions.
· Regular Inspection
Check the Sodium Battery monthly. Look at the voltage, inspect for leaks, and verify electrolyte levels. A multimeter can help track voltage fluctuations. Early detection of issues guarantees longevity. Remember, safety gear is essential during inspection.
· Temperature Control
Control room temperature for Na-Batt. A cool environment is best, between 20 and 25 degrees Celsius. High temperatures can reduce the battery’s life. Low temperatures can slow down the electrochemical reactions. Use a digital thermometer to monitor room temperature.
· Charge Level Monitoring
Monitor the charge level of the Sodium Battery. Never let the battery discharge below 30%. Overcharging can lead to reduced battery life. Undercharging can cause capacity loss. Use a BMS to keep an eye on charge levels.
· Cleaning Terminals
Clean the Na-Batt terminals regularly. Dust and corrosion can affect the battery’s performance. A clean, soft cloth can remove dust. A mixture of baking soda and water can clean corrosion. After cleaning, apply petroleum jelly to prevent future corrosion.
· Avoid Deep Discharge
Avoid deep discharge of the Sodium Battery. Deep discharge means draining the battery to 0%. It can lead to capacity loss. Instead, aim to keep charge levels above 30%. Regular monitoring and proper use ensure the battery’s health.
· Battery Recycling
Sodium Battery, known as Na-B, needs proper recycling. After 3000 charge cycles, deliver Na-B to certified recycling facilities. They extract valuable elements like nickel and cobalt. Safe recycling reduces waste. Always use trusted sources for recycling.
· Avoid Overcharging
Overcharging can harm a Sodium Battery. Keep an eye on the charging cycle. Stop charging once full capacity, typically 3.6 volts, is reached. An overcharged battery can heat up. Overheating might damage the battery’s lifespan.
· Use Approved Chargers
An approved charger is crucial for Sodium Battery health. Chargers must meet the required DC output of 3.6 volts. Unapproved chargers can cause harm. Incorrect voltage input can affect the battery’s performance.
· Avoid Short Circuits
Short circuits can harm your Sodium Battery. Always check the battery terminals. Clean terminals prevent short circuits. Regular inspections of the battery setup help. Short circuits can lead to battery damage.
· Regular Capacity Test
Capacity testing keeps your Sodium Battery in check. A capacity test every six months is advised. Testing ensures the battery delivers its rated output. A capacity of 3000 charge cycles should be maintained. Regular testing aids in optimal performance.
· Battery Ventilation
Proper ventilation is important for Sodium Battery. Heat can cause damage. Batteries should be kept in cool places. High temperatures might affect the battery’s overall performance. Good ventilation ensures a healthy battery life.
Energy Storage and Sodium Batteries!

The role of sodium batteries in energy storage
· Peak Shaving
Sodium batteries (NaBs) can manage high energy demand. For example, during mid-day, energy use soars. NaBs absorb excess power. Later, NaBs release stored energy during peak hours. The goal is to smooth energy demand and cut strain on power plants.
· Load Leveling
For energy flow, NaBs play a big role. When energy production exceeds usage, NaBs store the surplus. At times of low energy production, they discharge the stored power.
· Grid Balancing
NaBs contribute to grid stability. If power supply and demand mismatch, the NaBs can adjust. They can either store excess power or provide additional power. This function of NaBs ensures a balanced power grid.
· Off-Grid Power
In remote areas, grid connection may not exist. Here, NaBs can serve as standalone power sources. They can store power from solar or wind sources. The stored power can be used when needed, ensuring continuous power supply.
· Renewable Integration
NaBs facilitate the integration of renewable energy. They can store energy generated from renewable sources like solar and wind. During periods of low renewable generation, NaBs provide stored power, thereby facilitating green energy use.
· Backup Power
During power outages, NaBs serve as reliable backup. They can quickly supply stored energy to essential devices. Hospitals and data centers, for instance, benefit from this feature of NaBs.
· Demand Response
NaBs can respond to changes in power demand. They can supply more power during high demand periods. During low demand, they store excess power. This feature enhances the reliability of power systems.
· Frequency Regulation
Frequency stability is vital for power grid health. NaBs can assist in maintaining this. By absorbing or supplying power, NaBs can help keep frequency within safe limits.
· Spinning Reserve
NaBs can act as a ‘spinning reserve’. In case of sudden power loss, they can provide immediate power.
· Voltage Support
Maintaining correct voltage is crucial. NaBs can aid in this by adjusting power output. They can either absorb excess power or supply power as needed. This ensures a steady voltage, essential for the safety and efficiency of electrical devices.
Potential of sodium batteries in renewable energy storage
· Solar Power
A sodium battery (SB) works well with solar power. When sunlight hits solar panels, energy gets converted to electricity. SB stores this energy efficiently. That’s due to high capacity and cycling stability. Later, when the sun’s not shining, SB releases energy. It’s a reliable energy storage battery system, 24 hours a day.
· Wind Power
Wind turbines generate power. SB makes wind energy more useful. Wind is inconsistent but SB is not. It stores power when the wind is strong. When the wind stops, SB provides steady energy. So, wind power becomes dependable because of SB.
· Hydro Power
Hydroelectricity harnesses water flow for power. But, can SB help here? Absolutely. During peak production, SB captures excess energy. When production dips, SB fills in the gaps. It smooths out supply, making hydro power more predictable.
· Geothermal Energy
The earth gives off heat, called geothermal energy. Sodium batteries use this energy well. They charge when geothermal plants produce power.
When power production lowers, SB gives stored energy back. So, SB makes geothermal energy reliable around the clock.
· Tidal Power
Tides are a great energy source. They’re predictable and SB loves predictability. During high tide, tidal generators produce power. SB stores it. At low tide, SB releases stored energy. So, tidal energy is available, rain or shine, thanks to SB.
· Bioenergy
Bioenergy comes from organic materials. Bioenergy plants create power and SB stores it. Fluctuations in power production don’t matter. SB evens out supply.
· Hydrogen Storage
Hydrogen is a promising fuel. However, it’s tricky to store. Enter SB. Hydrogen can be converted to electricity. SB can store that power. So, SB solves a big problem for hydrogen storage.
· Islanding
Islanding happens when a part of the grid works independently. SB can help here too. The independent part can have its own SB. It stores power when it’s abundant, and then supplies it when it’s scarce.
Conclusion
We’ve traversed the landscape of sodium battery technology together. We’ve uncovered its workings, its potential applications, and glimpsed its future implications. Sodium batteries stand poised to revolutionize our energy paradigm. The baton is now in your hands.
Navigate to BUZZUP.LEIZI to remain up-to-date on the latest in energy storage. Engage with the thrilling sphere of pioneering tech. Let’s collaboratively pave the path to a more sustainable, cleaner tomorrow with sodium batteries.


